Cranberry prophylaxis of recurrent urinary tract infection in infants has proven effective in an experimental adult model. There are few data on its efficacy, safety and recommended dose in the paediatric population.
MethodsA controlled, double-blind Phase III clinical trial was conducted on children older than 1 month of age to evaluate the efficacy and safety of cranberry in recurrent urinary tract infection. The assumption was of the non-inferiority of cranberry versus trimethoprim. Statistical analysis was performed using Kaplan Meier analysis.
ResultsA total of 85 patients under 1 year of age and 107 over 1 year were recruited. Trimethoprim was prescribed to 75 patients and 117 received cranberry. The cumulative rate of urinary infection associated with cranberry prophylaxis in children under 1 year was 46% (95% CI; 23–70) in children and 17% (95% CI; 0–38) in girls, with effectiveness at doses inferior to trimethoprim. In children over 1 year-old cranberry was not inferior to trimethoprim, with a cumulative rate of urine infection of 26% (95% CI; 12–41). The cranberry was well tolerated and with no new adverse effects.
ConclusionsOur study confirms that cranberry is safe and effective in the prophylaxis of recurrent urinary tract infection in infants and children. With the doses used, their efficiency is not less than that observed for trimethoprim among those over 1 year-old. (Clinical Trials Registry ISRCTN16968287).
La profilaxis con arándano americano de la infección de orina recurrente infantil se ha mostrado eficaz en el modelo experimental del adulto. Existen pocos datos sobre su eficacia, seguridad y dosis recomendadas en la población pediátrica.
MétodosSe desarrolla un ensayo clínico controlado, doble ciego en fase iii en niños mayores de un mes de edad para evaluar la eficacia y seguridad del arándano americano en la infección urinaria recurrente infantil. Se parte del supuesto de no inferioridad del arándano americano frente a trimetoprima. El análisis estadístico se realiza mediante un análisis de Kaplan Meier.
ResultadosSe reclutan 85 pacientes menores de un año de edad y 107 mayores de un año. Setenta y cinco pacientes reciben arándano y 117 trimetoprima. El porcentaje acumulado de infección de orina asociado a la profilaxis con arándano en menores de un año fue de 46% (IC 95%: 23-70) en niños y del 17% (IC 95%: 0-38) en niñas, con eficacia a las dosis utilizadas inferior a trimetoprima. En los niños mayores de un año de edad el arándano se mostró no inferior a trimetoprima, con un porcentaje acumulado de infección de orina de 26% (IC 95%: 12-41). El arándano americano fue bien tolerado, no registrándose efectos adversos.
ConclusionesNuestro estudio confirma que el arándano americano es seguro y eficaz en la profilaxis de infección urinaria recurrente en lactantes y niños. Con las dosis utilizadas su eficacia no es inferior a la observada para trimetoprima entre los mayores de un año de edad (Clinical Trials Registry ISRCTN16968287).
Although controversial, current evidence supports the use of long-term low-dose antibiotic treatment to control recurrent urinary tract infections (UTIs), with the number needed to treat (NNT) to prevent one UTI being 1.9.1.1 However, one of the main problems in prescribing long-term low-dose antibiotics is the increase in bacterial resistance to antibiotics, and the effects of such treatment on the selection of multidrug-resistant bacteria in the flora. For several decades, cranberry has been used to prevent recurrent UTI in adults.1 Most studies have focused on populations at higher risk of developing UTIs, such as pregnant women or the elderly. All of them have concluded that while the effects of cranberry seem to be beneficial, the optimal dosage is unknown, as is its potential usefulness in the paediatric age group, and there is little data on its safety in the latter population. Urinary tract infections are relatively frequent in childhood, and are found in 8% of girls and 2% of boys. The rate of recurrent UTI following pyelonephritis is of up to 20%.2 While the effectiveness of antibiotic prophylaxis for the prevention of UTIs has not been demonstrated, the concept has biological plausibility.3,4 Some studies2,5 seem to show that antibiotic prophylaxis has no impact on the incidence of renal scars, even in patients with high-grade reflux, which calls its usefulness into question. Other studies6 show that paediatric patients with recurrent UTIs undergoing prophylactic treatment with low-dose trimethoprim-sulfamethoxazole experience a 6% decrease in the risk of UTI compared to placebo (95% CI, 1–11%). On the other hand, prophylaxis with cephalosporins is associated with extended-spectrum beta-lactamase-producing or multidrug-resistant uropathogens, a fact on which some authors7 base their recommendation of prophylaxis with trimethoprim, which is associated with a lower decrease in antimicrobial susceptibility.
Our study assessed the efficacy and safety of cranberry in the paediatric population for the prophylaxis of recurrent urinary tract infections. Our assumption was that treatment with cranberry is not inferior to other prophylactic interventions of proven efficacy, such as trimethoprim.
MethodsOur study was a randomised double-blind phase III clinical trial with two treatment arms: glucose syrup with 3% cranberry extract, and trimethoprim. For a period of two years, we recruited children from 1 month to 13 years of age that received care at the paediatric nephrology and urology departments of our hospital. The maximum duration of follow-up for the patients in the sample was one year. The trial was approved by the local ethics committee, and we obtained a written informed consent from the parents of all participants.
The inclusion criteria were a history of recurrent UTI (more than 2 episodes of infection in the past 6 months), associated or not to vesicoureteral reflux of any grade. The exclusion criteria were the concurrent presence during episodes of UTI of other infectious diseases, metabolic disorders, chronic kidney failure, kidney stones, or liver failure; allergy or intolerance to any components of cranberry or trimethoprim; the presence of blood dyscrasias; or the express desire of the legal guardian that the child not participate in the study.
Once a patient was selected and the signed informed consent was obtained for participation in the study, he or she was assigned a record number. Once the researcher included a patient in the study, the researcher gave the parents a card with the corresponding identification number; this card was presented at the hospital pharmacy to retrieve the assigned treatment, which was masked and labelled according to the regulations of the Agencia Española del Medicamento (Spanish Agency of Medicines).
The cranberry extract was supplied by a registered brand. The treatments were prepared by suspending 3% cranberry extract in glucose syrup, and suspending trimethoprim in glucose syrup at a concentration of 8mg/mL. We did a characterisation of the cranberry extract to verify the amount of proanthocyanidins administered to patients and determine the concentration of the remaining polyphenolic fractions.8
The experimental group received a nightly 0.2mL/kg dose of cranberry syrup. The standard treatment group received a nightly 0.2mL/kg dose of a colour-masked suspension of trimethoprim, for which we added the colourant CC-1000-WS (E-120; Chr-Hansen) at a concentration of 0.1%, which was within the allowed 100ppm of carmine. The dose had to be administered before supper, and a full dose had to be given again if vomiting occurred within 30min from the administration of the first dose.
Our study followed the intention to treat principle at all times.
The follow-up of patients consisted in periodic checkups every 2 months or on demand by the patient whenever symptoms called for it. The patient was instructed to perform a urine culture whenever he or she had fever, urinary symptoms, vomiting or weight loss. Urinary tract infections were confirmed by pathological findings in urine sediment (>20 WBC per field) and urine culture (>100000CFU/mL) from specimens obtained in a clean catch or a urine collection bag following disinfection of the urethral meatus with chlorhexidine, or more than 10000CFU/mL if the sample was obtained through a catheter.
A urine culture was always performed before initiating any type of antibiotic treatment. We documented the assigned intervention number, clinical and laboratory data for each of the scheduled check-up visits of the patient, and the periodic urine culture results. The effectiveness of the interventions was assessed based on the time spent in the study without recurrence of UTI. We documented the time at which recurrence occurred, at which point the participation of the patient in the study was terminated.
For sample size determination, we estimated the risk of recurrent UTI in the first year of antibiotic prophylaxis at 20%.2,9 We hypothesised that cranberry prophylaxis would be equivalent to trimethoprim prophylaxis. We set an equivalence margin of ±10%. Calculations were made for an alpha error of 5% and a power of 80%. With these values, we obtained a sample size of 109 patients per group.
Avoidance of biasThe possibility of obtaining false positive or negative results in the urine cultures performed to monitor the patient led us to consider the possibility of an information bias. It is possible to obtain a negative culture result in patients that do have a UTI if they have undergone antibiotic treatment prior to the collection of urine. Thus, it was underscored that a sample for urine culture should be collected, preferably by catheter, from any patient presenting with fever before initiating any prescribed antibiotic treatment. Positive urine culture results may be obtained due to improper collection technique and urine contamination; cultures positive for more than one microorganism when the urine sediment was normal were considered contaminated and repeated.
We also tried to prevent information bias by data cleansing and using electronic databases with systems to restrict incorrect input (dates).
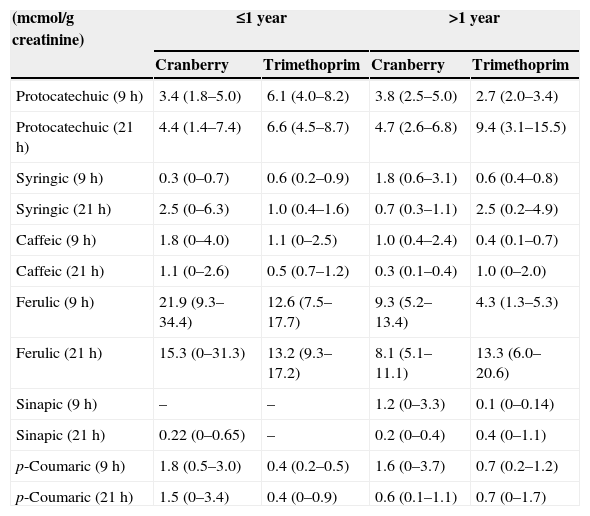
Determination of phenolic acids in urineSamples of urine collected at 9 am and 9 pm were tested for 6 dietary phenolic acids: protocatechuic acid, syringic acid, caffeic acid, ferulic acid, sinapic acid, and p-coumaric acid. The determinations were made at the Institute of Public Health and Clinical Nutrition in Kuopio, Finland. It is believed that some of these phenolic acids are products of the cleavage of procyanidins, flavonols and catechins found in the diet and in cranberries.10,11
In our statistical analysis we used the Kaplan–Meier estimator (survival analysis), and the occurrence of the event (UTI) marked the end of the follow-up period. Patients that withdrew voluntarily from the study or for reasons other than a UTI were considered excluded from further followup. We evaluated the Kaplan–Meier curves for the experimental intervention and the standard treatment. We compared the urinary excretion of polyphenols between groups with the independent-samples t test. We performed a linear regression analysis for the concentrations of the different polyphenolic components ingested in the cranberry syrup and the urinary excretion of phenolic acids.
For the noninferiority analysis, we considered the difference of the UTI prevalence between the two treatment arms (d), and calculated the standard error:
As the equivalence margins were estimated at ±0.15 (15%), δL=−0.10 and δU=0.10 for a zα value of 1.96.
We analysed the noninferiority of cranberry against trimethoprim by a unilateral hypothesis test:
H1L=pcranberry−ptrimethoprim>δL→H0L=pcranberry−ptrimethoprim≤δL
Which corresponds to zL=(d−δL)/SE≥Zα
H1U=pcranberry−ptrimethoprim>δUδ H0U=pcranberry−ptrimethoprim≥δU
Which corresponds to zL=(d−δL)/SE≤−Zα
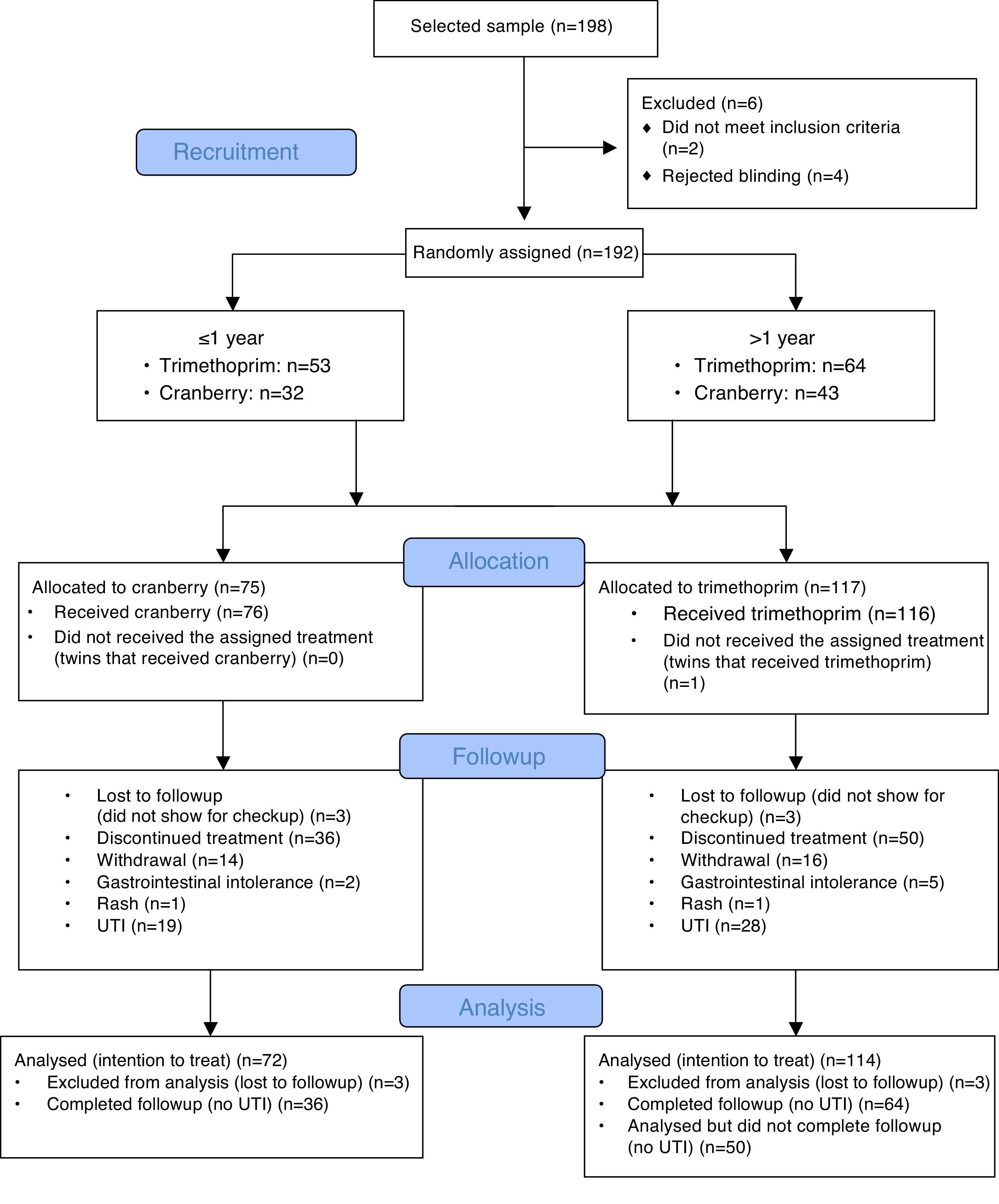
We studied 85 infants younger than 1 year, of which 53 received prophylaxis with trimethoprim and 32 prophylaxis with cranberry syrup. The study also included 107 children older than 1 year, 64 of which received trimethoprim prophylaxis and 43 cranberry prophylaxis.
In patients younger than 1 year, the cumulative rate of UTI associated with trimethoprim prophylaxis was 19% (95% CI, 4–35) in boys and 43% (95% CI, 18–68) in girls. In patients younger than 1 year, the cumulative rate of UTI associated with cranberry prophylaxis was 46% (95% CI, 23–70) in boys and 17% (95% CI, 0–38) in girls. For both sexes overall, the cumulative rate of UTI in infants that received trimethoprim was 28% (95% CI, 13–42) while this rate was 35% (95% CI, 17–52) in infants that received cranberry.
The overall cumulative rate of UTI in children older than 1 year was 35% (95% CI, 21–50) in those that received trimethoprim and 26% (95% CI, 12–41) in those that received cranberry. By sex, the cumulative rate of UTI in males that received trimethoprim was 33% (95% CI, 1.8–65) compared to 8% (95% CI, 0–26) in males that received cranberry.
The CONSORT diagram (Fig. 1) summarises the adverse events detected in children less and more than 1 year of age during the follow-up period. There was a remarkably low incidence of adverse reactions, and we did not detect any adverse events in infants less than 1 year of age. Table 1 shows the urinary excretion of phenolic acids in patients younger and older than 1 year in the trimethoprim and the cranberry groups, with no significant differences found in the excretion of phenolic acids between strata.
Urinary excretion of phenolic acids in patients receiving nightly prophylaxis with cranberry or trimethoprim.
| (mcmol/g creatinine) | ≤1 year | >1 year | ||
|---|---|---|---|---|
| Cranberry | Trimethoprim | Cranberry | Trimethoprim | |
| Protocatechuic (9h) | 3.4 (1.8–5.0) | 6.1 (4.0–8.2) | 3.8 (2.5–5.0) | 2.7 (2.0–3.4) |
| Protocatechuic (21h) | 4.4 (1.4–7.4) | 6.6 (4.5–8.7) | 4.7 (2.6–6.8) | 9.4 (3.1–15.5) |
| Syringic (9h) | 0.3 (0–0.7) | 0.6 (0.2–0.9) | 1.8 (0.6–3.1) | 0.6 (0.4–0.8) |
| Syringic (21h) | 2.5 (0–6.3) | 1.0 (0.4–1.6) | 0.7 (0.3–1.1) | 2.5 (0.2–4.9) |
| Caffeic (9h) | 1.8 (0–4.0) | 1.1 (0–2.5) | 1.0 (0.4–2.4) | 0.4 (0.1–0.7) |
| Caffeic (21h) | 1.1 (0–2.6) | 0.5 (0.7–1.2) | 0.3 (0.1–0.4) | 1.0 (0–2.0) |
| Ferulic (9h) | 21.9 (9.3–34.4) | 12.6 (7.5–17.7) | 9.3 (5.2–13.4) | 4.3 (1.3–5.3) |
| Ferulic (21h) | 15.3 (0–31.3) | 13.2 (9.3–17.2) | 8.1 (5.1–11.1) | 13.3 (6.0–20.6) |
| Sinapic (9h) | – | – | 1.2 (0–3.3) | 0.1 (0–0.14) |
| Sinapic (21h) | 0.22 (0–0.65) | – | 0.2 (0–0.4) | 0.4 (0–1.1) |
| p-Coumaric (9h) | 1.8 (0.5–3.0) | 0.4 (0.2–0.5) | 1.6 (0–3.7) | 0.7 (0.2–1.2) |
| p-Coumaric (21h) | 1.5 (0–3.4) | 0.4 (0–0.9) | 0.6 (0.1–1.1) | 0.7 (0–1.7) |
Sixty percent of the episodes of recurrent UTI were caused by Escherichia coli, and we found no significant differences between the two treatment groups. Of all patients that received trimethoprim, 33.3% had urine cultures positive for multidrug-resistant bacteria, compared to 22.9% of the patients that received cranberry.
For the whole sample, we found that ZL=2.04>Zα and ZU=−1.65>−Zα, so we can accept the hypothesis that treatment with cranberry is not inferior to treatment with trimethoprim. In infants less than 1 year of age, our results were ZL=0.69<Zα and ZU=−1.9>−Zα, so we had to accept that in infants treatment with cranberry at the administered doses is inferior to trimethoprim.
Of the patients less than 1 year of age, 13 had vesicoureteral reflux (VUR), four of them grade 2 or lower, and five grade 3 or higher, of which two and four, respectively, were treated with cranberry. We did not observe a difference in the recurrence of UTI among groups. Of the patients more than 1 year of age, 27 had RVU, 11 of them grade 2 or less, and 16 grade 3 or more, of which 3 and 8, respectively, were treated with cranberry. We did not observe any differences in UTI recurrence between the groups.
DiscussionOur study confirms that the use of cranberry is safe in infants and children. The efficacy of cranberry is not inferior to that of trimethoprim for the prophylaxis of recurrent UTI in children, although its efficacy in infants less than 1 year of age can be considered inferior to that of trimethoprim at the doses we administered.
In 1984 Sobota12 observed that cranberry extract interferes with the adherence of p-fimbriated E. coli to the epithelium, demonstrating that this may be one of the main mechanisms underlying the anti-adhesion activity of cranberry. The fructose contained in many fruit juices, including cranberry juice, may also inhibit the adherence of type 1 fimbriae.13 However, adhesion mediated by p-fimbriae has been associated with the specific lectin α-Gal(1–4)β-Gal found in the urothelium, the binding of which is not inhibited by the addition of fructose.14,15 Foo et al.16 identified A-type proanthocyanidin trimers and procyanidin dimers as being responsible for the anti-adhesion activity of cranberry. They are polyphenolic flavonols that are not found in other substances rich in polyphenols, such as green tea or chocolate. The cranberry extract used in our trial contained 22% of A-type proanthocyanidins, which is relevant for the future comparison of our results with other studies given the wide variability in the concentration of proanthocyanidins found in different cranberry extracts.17,18
Two studies assessed the effectiveness of cranberry in children with neurogenic bladder managed by clean intermittent catheterisation.19,20 The first study had a single-blind cross-over design and included 40 children, and the second was a double-blind placebo-controlled study and included 15 children. Both studies concluded that cranberry concentrate was no more efficacious than placebo in controlling UTI in patients with neurogenic bladder. However, neither study characterised the polyphenolic content of the cranberry preparations used.
McMurdo et al.21 conducted a randomised double-blind trial with cranberry or trimethoprim in women at risk of recurrent UTI. The authors observed that the risk of UTI recurrence was 60% higher in patients receiving cranberry, although the finding was not statistically significant. A clinical trial carried out by Uberos et al.22 demonstrated the noninferiority of cranberry against trimethoprim in a sample of children younger than 14 years; this study did not stratify the sample by age and did not take into account the relationship between cranberry intake and urinary excretion of phenolic acids. The systematic review published by Jepson1 of a total of 10 studies (1049 patients) concluded that cranberry, compared to placebo or control treatment, significantly decreased the incidence of UTI over a 12 month period (RR, 0.65; 95% CI, 0.46–0.90). Cranberry was more effective in reducing the incidence of UTI in women with recurrent UTIs than in men, older women or patients requiring catheterisation.
Previous studies23 have demonstrated the beneficial effect of cranberry in the prevention of UTI in women, with a lower absolute risk of UTI relative to placebo. In a placebo-controlled clinical trial in children older than 3 years, Ferrara et al.24 demonstrated that cranberry prevents the recurrence of symptomatic UTIs. Our study is the first randomised controlled double-blind trial that demonstrates the safety of cranberry in infants and children. Howell et al.25 found evidence that the anti-adhesion activity of cranberry is greater when the amount of proanthocyanidins administered in the cranberry preparation is higher than 18mg. Since no data were available on the use of cranberry in infants, for our study we decided to extrapolate from the adult dosage and administer 0.2mL of the syrup per kilogram of body weight. Our results show that cranberry is not inferior to trimethoprim when the dose of proanthocyanidins is above 18mg, which is the dose that would be administered per kilogram of body weight in a one-year old infant.
Phenolic acids are considered products of the cleavage of procyanidins, flavonols and catechins from dietary sources or cranberries.10,11 We found slightly higher levels of p-coumaric acid and ferulic acid at 9h, at the limit of statistical significance, in the group of infants treated with cranberry. We found no differences in the rest of the dietary phenolic acids analysed. In our study, we determined the phenolic acids that are considered to originate from dietary sources, that is, those whose urinary excretion is strongly influenced by diet. The urinary excretion of other phenolic metabolites that were not determined in this study may be affected not only by dietary factors, but also by the metabolism of endogenous molecules such as catecholamines.26,27 We analysed the concentration of cyanidins eliminated by the patients during their followup periods, and found values below the detection limits. 28 The phenolic metabolites in urine found to have the strongest association with cranberry intake are caffeic acid, dihydroferulic acid, p-coumaric acid, dihydroxybenzoic acid and 4-O-b-d-glucuronic acid.28
Since the anti-adhesion activity of cranberry is dose-dependent and the best results were obtained in patients more than 1 year of age in whom the administration of more than 18mg of proanthocyanidins was guaranteed, we believe that it is necessary to conduct other studies assessing the efficacy of different dosages of cranberry in infants less than 1 year of age.
FundingThis clinical trial was funded by the Fondo de Investigaciones Sanitarias (Health Research Fund) of the Instituto de Salud Carlos III, Madrid.
Conflicts of interestThe authors have no conflicts of interest to declare.
We want to thank Dr Tarja Nurmi of the University of Kuopio, Finland, for her guidance in the laboratory determinations performed for this study.
Please cite this article as: Fernández-Puentes V, Uberos J, Rodríguez-Belmonte R, Nogueras-Ocaña M, Blanca-Jover E, Narbona-López E. Eficacia y perfil de seguridad del arándano americano en lactantes y niños con infección urinaria recurrente. An Pediatr (Barc). 2015;82:397–403.