The perineal adhesive bag is the most commonly used method in our country for urine culture collection in infants, despite having a high risk of contamination and false-positive results. We aim to quantify both types of risks through a systematic review.
MethodsSearch updated in May 2014 in PUBMED, SCOPUS (includes EMBASE), IBECS; CINAHL, LILACS AND CUIDEN, unrestricted by language or date. Percentage of contaminated samples, false positives, sensitivity and specificity (with respect to catheterization or bladder puncture) were recorded.
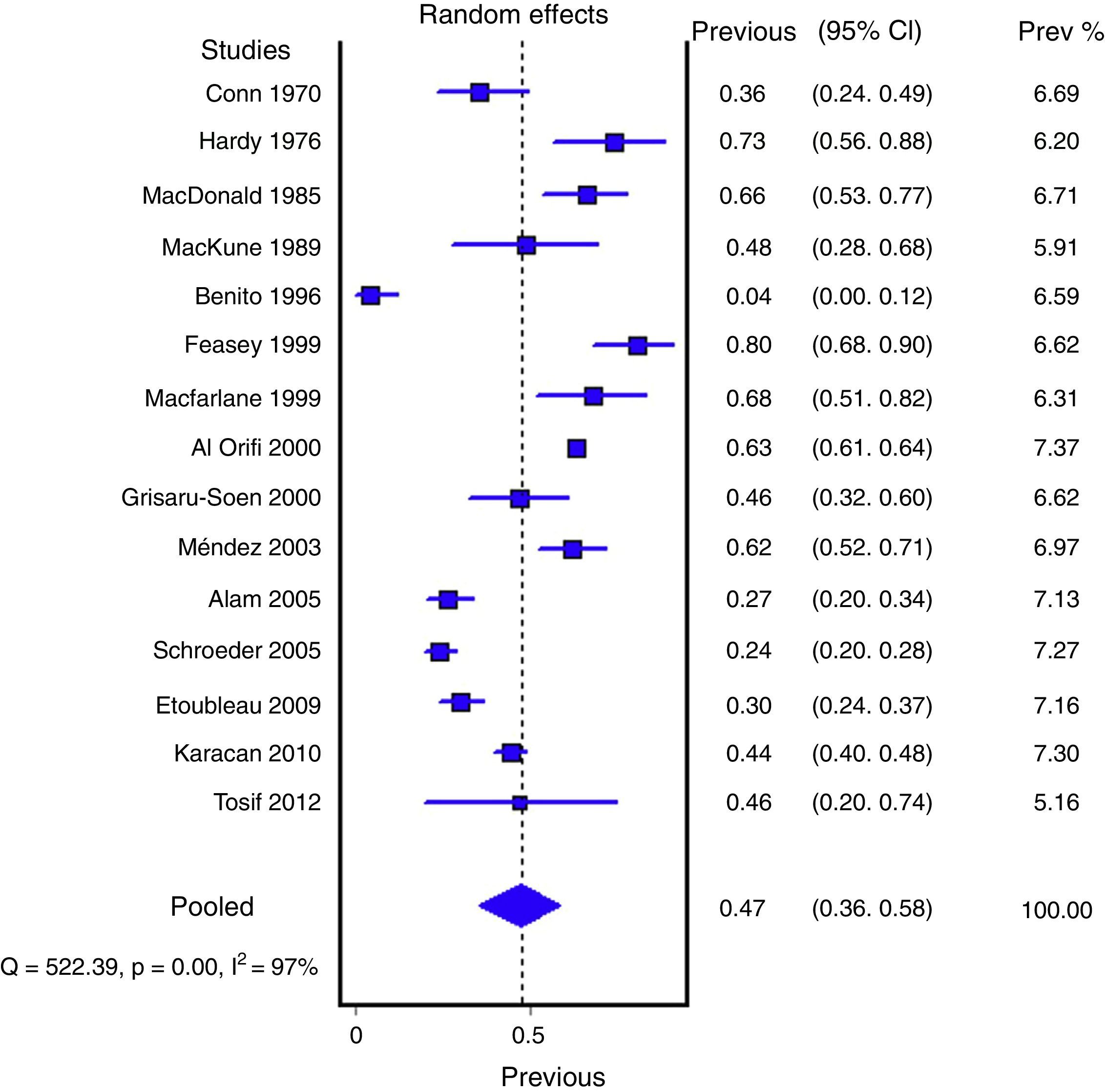
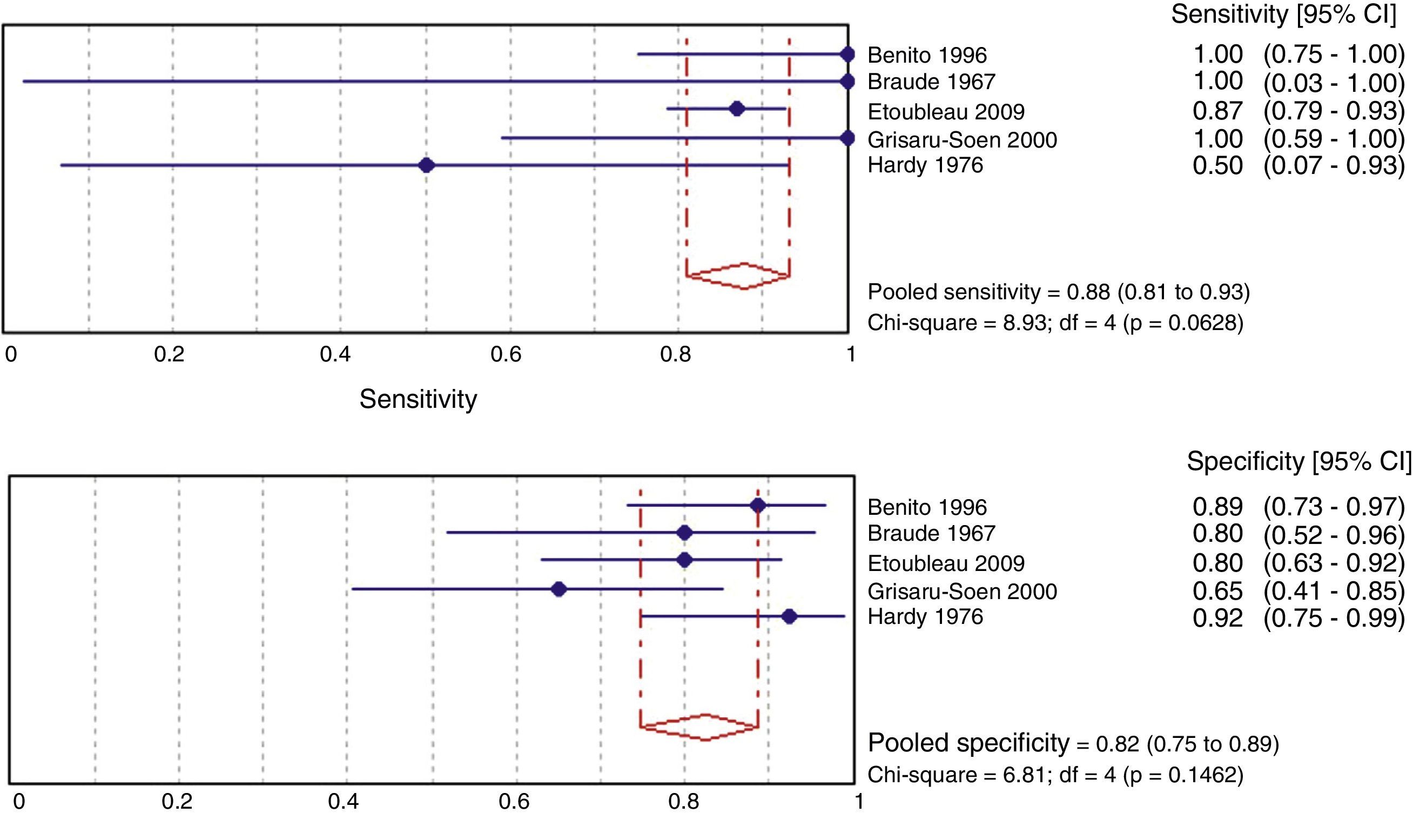
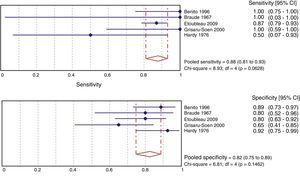
ResultsA total of 21 studies of medium quality (7659 samples) were selected. The pooled percentage of contaminated samples was 46.6% (15 studies; 6856 samples; 95% confidence interval [95% CI]: 35.6–57.8%; I2: 97.3%). The pooled percentage of false positives was 61.1% (12 studies; 575 samples; 95% CI: 37.9–82.2%; I2: 96.2%). Sensitivity (88%; 95% CI: 81–93%; I2: 55.2%) and specificity (82%; 95% CI: 75–89%; I2: 41.3%) were estimated in five studies, but without including contaminated samples.
ConclusionThe perineal adhesive bag is not a valid enough method for urine culture collection, because almost half of all samples are contaminated, and two out of three positives are false. Although these estimates are imprecise, because of their great heterogeneity, they should be considered when choosing the method of urine collection. The estimates of sensitivity and specificity are not applicable because they do not take into account the risk of contamination.
La bolsa adhesiva perineal es el método más usado en nuestro medio para la recogida de orina para cultivo en el lactante, a pesar de que presenta un alto riesgo de contaminación y de resultados falsos positivos. Nos proponemos cuantificar ambos riesgos a través de una revisión sistemática.
MétodosBúsqueda actualizada a mayo del 2014 en PUBMED, SCOPUS (incluye EMBASE), IBECS; CINHAL, LILACS Y CUIDEN, sin límites de idioma ni tiempo. Se extrajeron porcentajes de orinas contaminadas, falsos positivos, sensibilidad y especificidad (respecto cateterismo o punción vesical).
ResultadosSe seleccionaron 21 artículos de calidad media (7.659 muestras). El porcentaje agrupado de orinas contaminadas fue del 46,6% (15 estudios; 6.856 muestras; intervalo de confianza del 95% [IC del 95%], 35,6 a 57,8%; I2: 97,3%). El porcentaje agrupado de falsos positivos fue del 61,1% (12 estudios; 575 muestras; IC del 95%, 37,9 a 82,2%; I2: 96,2%). En 5 estudios se pudieron estimar la sensibilidad (88%; IC del 95%, 81 a 93%; I2: 55,2%) y especificidad (82%; IC del 95%, 75 a 89%; I2: 41,3%), aunque en los recuentos no se incluyeron orinas contaminadas.
ConclusiónLa bolsa adhesiva perineal no es un método suficientemente válido para cultivo de orina porque casi la mitad resultarán contaminados y de los positivos 2 de cada 3 serán falsos. Aun siendo estimaciones imprecisas, por su gran heterogeneidad, deben ser tenidas en cuenta en la elección del método de recogida de orina. Las estimaciones de sensibilidad y especificidad no son aplicables por no considerar el riesgo de contaminación.
Urinary tract infection (UTI) in paediatric patients is one of the most frequent causes of visits in the health care system, in Primary Care as well as in the emergency room, and it is a frequent cause of hospitalisation. In children, urinary tract infection symptoms are usually unspecific, such as fever without a source, food rejection or failure to thrive. That is why diagnostic confirmation with a urine culture is particularly important. Diagnostic methods must be quick and sensitive enough to facilitate prompt treatment and lower mortality rates, but specific enough to avoid unnecessary complementary tests and therapies.
Currently, we have several techniques for the collection of urine samples from children. When the child has bladder control, the technique usually employed is the collection of urine from the urinary stream. In children who do not have bladder control, the most widely used method is the perineal adhesive bag, even though samples collected with this technique have a high risk of contamination or false positives. Samples collected through bladder catheterisation or suprapubic aspiration have a lower risk of contamination, but these are invasive, potentially risky techniques. Therefore, they are used as confirmatory tests or in emergency situations in which an immediate diagnosis or treatment is required.1,2 The use of sterile compresses, a technique employed in other countries, is uncommon in Spain.
Several technical reports and clinical practice guidelines mention studies that show the limitations of the perineal bag for urine culture collection.1,3–5 However, no review studies have as yet quantified the principle problems associated with this sampling technique: the high risk of contamination and false positives. Our objective is to perform a systematic review to estimate the validity of samples collected with a perineal bag as a basis for clinical practice guidelines.
Material and methodsBibliographic searchThe PUBMED, SCOPUS (including PUBMED and EMBASE) CINHAL, IBECS and CUIDEN databases were searched for articles published up to May 2014, with no restrictions on type of study, language, or year of publication.
For PUBMED, SCOPUS and CINHAL, 2 search strategies were employed: 1 extensive search using the string (“infant”[MeSH Terms] OR “child”[MeSH Terms]) AND (“Urinary tract infections”[MeSh] OR (“Urine” AND “Culture”) OR (Urinary AND Infection)) AND (“Specimen Handling”[MeSh] OR Specim* OR “Urine Specimen Collection”[Mesh] OR “Bag” OR “Bags”) AND (“Urine/microbiology”[Mesh] OR “Contamination” OR “false positive” OR “Diagnosis”[Mesh] OR “Sensitivity and Specificity”[Mesh]”; and 1 simplified search using (“infant OR child”) AND “urine” AND “specimen handling”. In IBECS and CUIDEN, the equivalent terms in Spanish were employed. The references from the recovered studies were examined to identify other undetected studies.
A total of 935 references were identified in the searches performed after the exclusion of duplicates. The titles and summaries of these references were reviewed and 40 full-text articles were selected. The references from the recovered articles were examined and 7 supplementary articles unidentified in the initial searches were found. The 47 recovered studies were examined to assess their inclusion in the review. The entire search and review process was performed in duplicate. Disagreements on criteria among the reviewers were resolved by consensus.
Study selection criteriaThe studies selected were diagnostic test assessments, unlimited by design, language and date, that analysed urine samples for culture collected using a perineal bag from ambulatory or hospitalised paediatric patients of both genders with suspicion or at risk of UTI (excluding studies in healthy children). The studies had to include the number or percentage of contaminated samples (according to specified microbiological criteria) or false positives (positive urine culture with bag unconfirmed by urine cultures performed via suprapubic aspiration or bladder catheterisation). Studies with data that enabled the estimation of the sensitivity and specificity of urine cultures collected via perineal bag in comparison with urine cultures collected via bladder catheterisation or suprapubic aspiration were also included.
Data extraction and quality assessmentFrom each study, information on sample size, country, area (hospital, emergency department or ambulatory patient), patient characteristics (age and sex), inclusion and exclusion criteria, assessed tests, assessment criteria, data (contaminated samples, true positives, false positives, false negatives and true positives), other results, quality and limitations were extracted in duplicate.
The quality criteria assessed were:
- –
Well-defined test or strategy: the information provided facilitates reproducibility.
- –
Valid assessment criterion: enables the culture to be classified correctly as contaminated, negative or positive.
- –
Representative sample: similar to the patient population found in clinical practice (no biased selection of positive samples or exclusion of contaminated samples).
- –
Independent comparison: the test assessment or interpretation and the assessment criterion are independent and objective.
- –
Diagnostic verification and incorporation bias control: the first one occurs when the reference test is performed with higher or lower likelihood according to the result of the diagnostic test to be assessed, and the second occurs when the diagnostic test result is part of the assessment criterion (e.g., positive urine cultures collected via bag are considered UTIs).
- –
Correct analysis: enough information is provided so that unbiased percentages of contaminated urines, false positives, sensitivity and specificity can be calculated.
A grouped analysis was performed for the percentages of contaminated and false positives, assuming random-effects models. The calculations and charts were generated with the MetaXL utility for Excel (EpiGear International Pty Ltd). For the calculation of grouped validity measures (sensitivity and specificity), the programme MetaDiSc (ES) v.1.1.1 was employed.6 Heterogeneity indicators (Cochrane's Q & I2) and 95% confidence intervals (95% CI) were estimated for all the parameters. Tests for heterogeneity (Dersimonian and Laird) publication bias (Begg and Egger) and sensitivity (excluding individual studies) were performed. Analyses of subgroups, meta-regressions (geographical area and representative sample), Galbraith charts, cumulative and sensitivity charts were performed.
ResultsOut of the 47 full-text articles examined, 21 that complied with the inclusion criteria were selected.7–27 The purpose of most of the studies included was to determine the validity of the perineal bag versus other techniques through different estimators. In 15 studies.7,12–14,16–18,21,22,24–27 contaminated samples were estimated, and in 129,15–17,19,20,23,25 false positives were estimated. The validity related to an external reference pattern was determined in only 5 studies.9,11,13,16,17 One article was excluded because it analysed urine samples by a test strip and not by culture.28
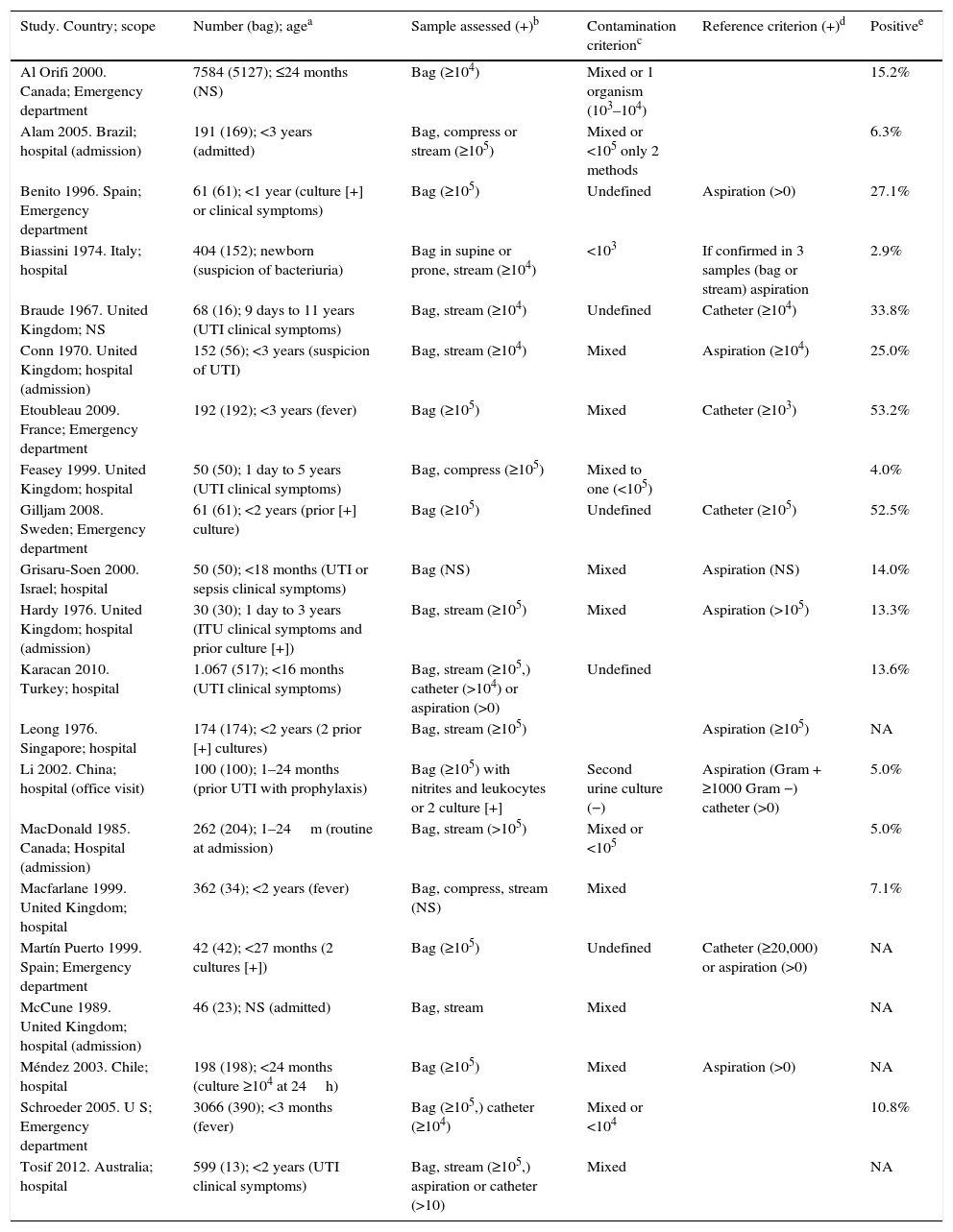
Table 1 shows the simplified characteristics of the 21 studies included in the meta-analysis. The studies were carried out in different countries, but their assessment methods and characteristics were very similar. They included a total of 14,759 patients, 7659 with urine bag samples. The ages of the children included in the study are similar, between 1 day and 3 years of age, except for some studies that included some older patients.11,14 The children included had clinical symptoms (most of them with fever without source), suspicion of UTI, or prior positive cultures using a non-sterile urine collection technique. Gender-specific variables are specified in 5 studies,8,11,12,17,23 with a discrete higher frequency of girls.
Characteristics of the studies included in the meta-analysis.
| Study. Country; scope | Number (bag); agea | Sample assessed (+)b | Contamination criterionc | Reference criterion (+)d | Positivee |
|---|---|---|---|---|---|
| Al Orifi 2000. Canada; Emergency department | 7584 (5127); ≤24 months (NS) | Bag (≥104) | Mixed or 1 organism (103–104) | 15.2% | |
| Alam 2005. Brazil; hospital (admission) | 191 (169); <3 years (admitted) | Bag, compress or stream (≥105) | Mixed or <105 only 2 methods | 6.3% | |
| Benito 1996. Spain; Emergency department | 61 (61); <1 year (culture [+] or clinical symptoms) | Bag (≥105) | Undefined | Aspiration (>0) | 27.1% |
| Biassini 1974. Italy; hospital | 404 (152); newborn (suspicion of bacteriuria) | Bag in supine or prone, stream (≥104) | <103 | If confirmed in 3 samples (bag or stream) aspiration | 2.9% |
| Braude 1967. United Kingdom; NS | 68 (16); 9 days to 11 years (UTI clinical symptoms) | Bag, stream (≥104) | Undefined | Catheter (≥104) | 33.8% |
| Conn 1970. United Kingdom; hospital (admission) | 152 (56); <3 years (suspicion of UTI) | Bag, stream (≥104) | Mixed | Aspiration (≥104) | 25.0% |
| Etoubleau 2009. France; Emergency department | 192 (192); <3 years (fever) | Bag (≥105) | Mixed | Catheter (≥103) | 53.2% |
| Feasey 1999. United Kingdom; hospital | 50 (50); 1 day to 5 years (UTI clinical symptoms) | Bag, compress (≥105) | Mixed to one (<105) | 4.0% | |
| Gilljam 2008. Sweden; Emergency department | 61 (61); <2 years (prior [+] culture) | Bag (≥105) | Undefined | Catheter (≥105) | 52.5% |
| Grisaru-Soen 2000. Israel; hospital | 50 (50); <18 months (UTI or sepsis clinical symptoms) | Bag (NS) | Mixed | Aspiration (NS) | 14.0% |
| Hardy 1976. United Kingdom; hospital (admission) | 30 (30); 1 day to 3 years (ITU clinical symptoms and prior culture [+]) | Bag, stream (≥105) | Mixed | Aspiration (>105) | 13.3% |
| Karacan 2010. Turkey; hospital | 1.067 (517); <16 months (UTI clinical symptoms) | Bag, stream (≥105,) catheter (>104) or aspiration (>0) | Undefined | 13.6% | |
| Leong 1976. Singapore; hospital | 174 (174); <2 years (2 prior [+] cultures) | Bag, stream (≥105) | Aspiration (≥105) | NA | |
| Li 2002. China; hospital (office visit) | 100 (100); 1–24 months (prior UTI with prophylaxis) | Bag (≥105) with nitrites and leukocytes or 2 culture [+] | Second urine culture (−) | Aspiration (Gram + ≥1000 Gram −) catheter (>0) | 5.0% |
| MacDonald 1985. Canada; Hospital (admission) | 262 (204); 1–24m (routine at admission) | Bag, stream (>105) | Mixed or <105 | 5.0% | |
| Macfarlane 1999. United Kingdom; hospital | 362 (34); <2 years (fever) | Bag, compress, stream (NS) | Mixed | 7.1% | |
| Martín Puerto 1999. Spain; Emergency department | 42 (42); <27 months (2 cultures [+]) | Bag (≥105) | Undefined | Catheter (≥20,000) or aspiration (>0) | NA |
| McCune 1989. United Kingdom; hospital (admission) | 46 (23); NS (admitted) | Bag, stream | Mixed | NA | |
| Méndez 2003. Chile; hospital | 198 (198); <24 months (culture ≥104 at 24h) | Bag (≥105) | Mixed | Aspiration (>0) | NA |
| Schroeder 2005. U S; Emergency department | 3066 (390); <3 months (fever) | Bag (≥105,) catheter (≥104) | Mixed or <104 | 10.8% | |
| Tosif 2012. Australia; hospital | 599 (13); <2 years (UTI clinical symptoms) | Bag, stream (≥105,) aspiration or catheter (>10) | Mixed | NA |
Catheter: bladder catheterisation; stream: urinary stream; compress: sterile compress; NA: not applicable; NS: not stated; aspiration: suprapubic aspiration.
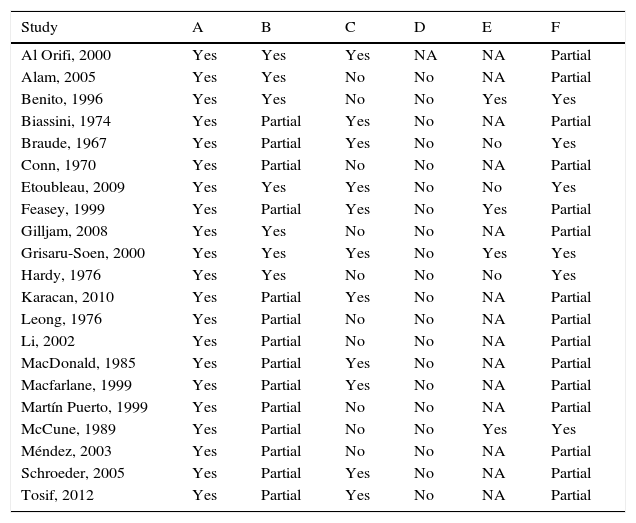
Table 2 shows the quality assessment of all the studies included in the meta-analysis. The assessment criteria employed for sample contamination or sample positivity/negativity is appropriate, although only one third of the studies use a complete reference pattern for all the samples. In half of the studies, the sample presents some type of bias, mainly in those that assessed sensitivity and specificity, since the analyses do not include contaminated samples. In other studies, the sample includes an over-representation of patients with previously positive urines. None of the studies detail procedures that guarantee the independent or blind assessment of the compared tests, although culture interpretation is a relatively objective test. The biased incorporation of bag samples with positive culture is common in several studies. Finally, all the studies included offer data that enable a correct analysis, although a complete validity assessment can be performed in only one quarter of studies.
Quality of the studies included in the meta-analysis.
| Study | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Al Orifi, 2000 | Yes | Yes | Yes | NA | NA | Partial |
| Alam, 2005 | Yes | Yes | No | No | NA | Partial |
| Benito, 1996 | Yes | Yes | No | No | Yes | Yes |
| Biassini, 1974 | Yes | Partial | Yes | No | NA | Partial |
| Braude, 1967 | Yes | Partial | Yes | No | No | Yes |
| Conn, 1970 | Yes | Partial | No | No | NA | Partial |
| Etoubleau, 2009 | Yes | Yes | Yes | No | No | Yes |
| Feasey, 1999 | Yes | Partial | Yes | No | Yes | Partial |
| Gilljam, 2008 | Yes | Yes | No | No | NA | Partial |
| Grisaru-Soen, 2000 | Yes | Yes | Yes | No | Yes | Yes |
| Hardy, 1976 | Yes | Yes | No | No | No | Yes |
| Karacan, 2010 | Yes | Partial | Yes | No | NA | Partial |
| Leong, 1976 | Yes | Partial | No | No | NA | Partial |
| Li, 2002 | Yes | Partial | No | No | NA | Partial |
| MacDonald, 1985 | Yes | Partial | Yes | No | NA | Partial |
| Macfarlane, 1999 | Yes | Partial | Yes | No | NA | Partial |
| Martín Puerto, 1999 | Yes | Partial | No | No | NA | Partial |
| McCune, 1989 | Yes | Partial | No | No | Yes | Yes |
| Méndez, 2003 | Yes | Partial | No | No | NA | Partial |
| Schroeder, 2005 | Yes | Partial | Yes | No | NA | Partial |
| Tosif, 2012 | Yes | Partial | Yes | No | NA | Partial |
A: well-defined test or strategy; B: valid assessment criterion; C: representative sample; D: independent comparison; E: diagnostic verification and incorporation bias control; F: correct analysis.
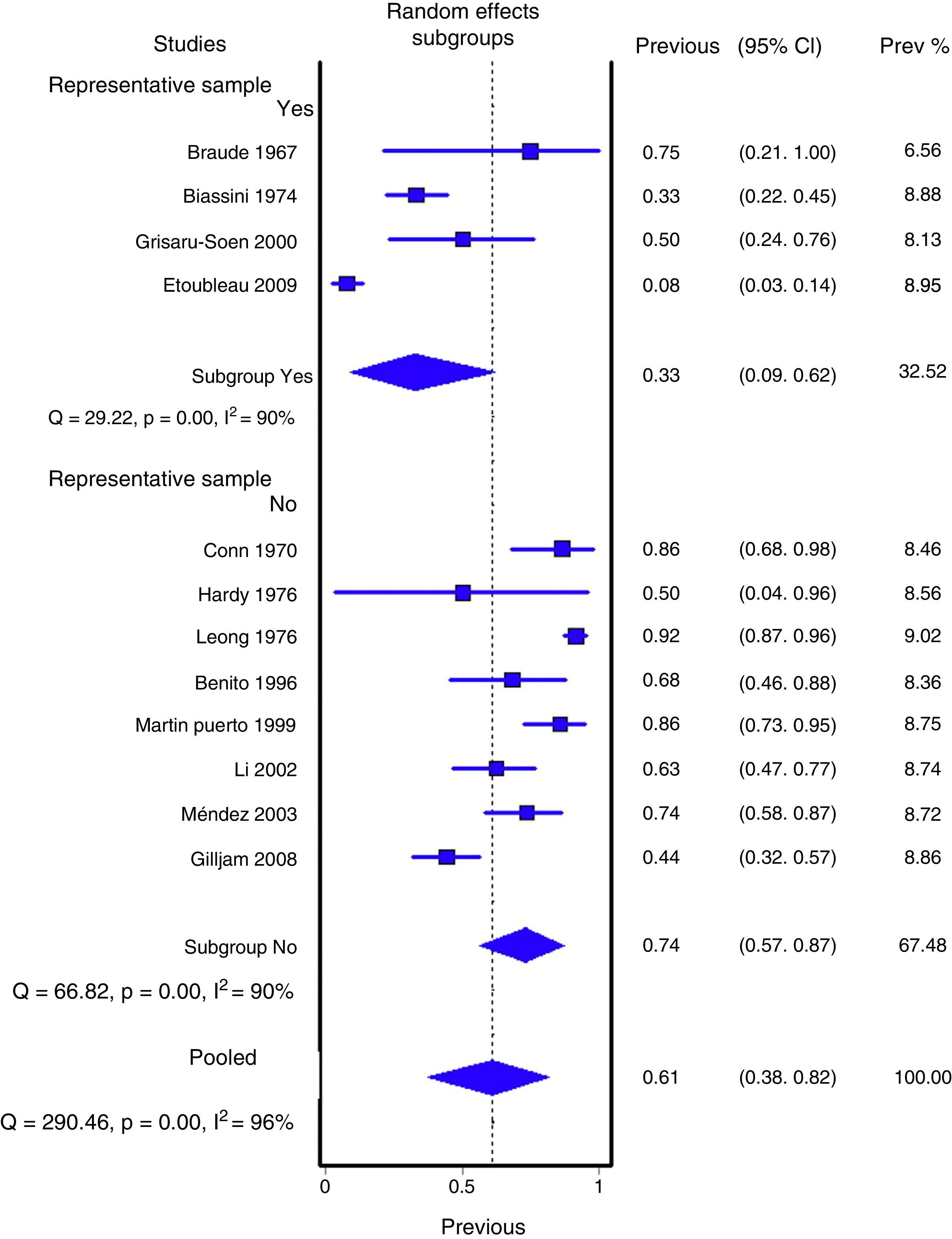
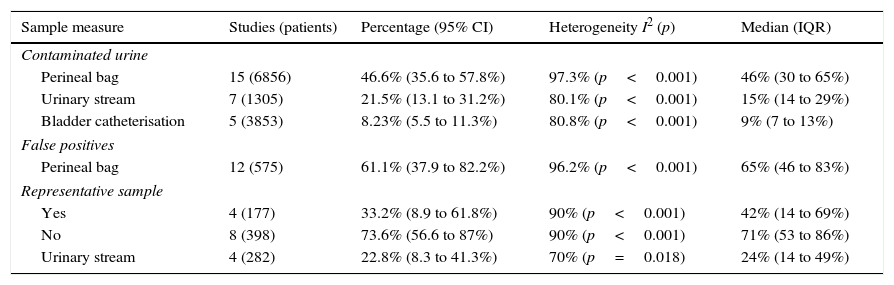
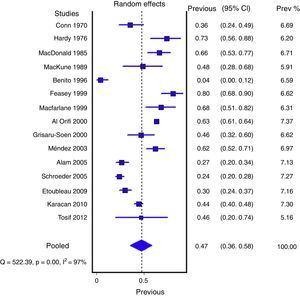
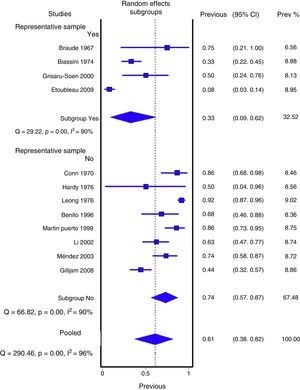
Figs. 1 and 2 show the meta-analysis of contaminated samples and false positives with perineal bag. Table 3 shows the pooled measurements for perineal bag and those of other samples analysed in the studies reviewed. For urine collected with the bag, the weighted percentage of contaminated urines is 46.6%, and for false positives 61.1%. In the meta-regression analyses, we found that studies with representative patient samples had a significantly lower percentage of false positives; therefore, we performed a meta-analysis by subgroups. All the analyses show important heterogeneity (I2>50%), although we were unable to identify a particular study that, when excluded, would reduce heterogeneity. The publication bias tests (Begg and Egger) showed no statistical significance.
Grouped meta-analysis results for contamination and false positives. Weighed percentages with their 95% confidence interval (95% CI), heterogeneity (I2), Dersimonian and Laird contrast (p), medians and interquartile ranges (IQR).
| Sample measure | Studies (patients) | Percentage (95% CI) | Heterogeneity I2 (p) | Median (IQR) |
|---|---|---|---|---|
| Contaminated urine | ||||
| Perineal bag | 15 (6856) | 46.6% (35.6 to 57.8%) | 97.3% (p<0.001) | 46% (30 to 65%) |
| Urinary stream | 7 (1305) | 21.5% (13.1 to 31.2%) | 80.1% (p<0.001) | 15% (14 to 29%) |
| Bladder catheterisation | 5 (3853) | 8.23% (5.5 to 11.3%) | 80.8% (p<0.001) | 9% (7 to 13%) |
| False positives | ||||
| Perineal bag | 12 (575) | 61.1% (37.9 to 82.2%) | 96.2% (p<0.001) | 65% (46 to 83%) |
| Representative sample | ||||
| Yes | 4 (177) | 33.2% (8.9 to 61.8%) | 90% (p<0.001) | 42% (14 to 69%) |
| No | 8 (398) | 73.6% (56.6 to 87%) | 90% (p<0.001) | 71% (53 to 86%) |
| Urinary stream | 4 (282) | 22.8% (8.3 to 41.3%) | 70% (p=0.018) | 24% (14 to 49%) |
In two studies8,17 the data from boys and girls was estimated separately, showing a higher risk of contaminated urine in boys (38.9% vs 29.2%; odds ratio [OR] 1.53; 95% CI: 1.36–1.72). The same was done for false positives in 4 studies, and no significant differences were found (OR: 0.96; 95% CI: 0.25–3.65). For samples collected via urinary stream and bladder catheterisation, the risks of contaminated urine or false positives are clearly lower.
Fig. 3 shows the meta-analysis for validity indicators of culture with perineal bag in comparison with the sterile technique. The weighted specificity and sensitivity are 82% and 88%, respectively. These estimates do not present heterogeneity.
DiscussionAlthough the use of urine collection for culture via perineal bag is widespread, the available evidence indicates that it is not a very valid method due to the high risk of contaminated samples and false positives. The studies retrieved from the bibliographic search, however, are extremely heterogeneous; therefore, these findings could be inexact. Although the heterogeneity observed could be unacceptable for relative risk measurements, it is common to find rates of over 90% in meta-analyses of prevalence studies.29–35 In fact, in risk meta-analyses, the baseline prevalence differences between studies, which are partially compensated by the relative measurements, are one of the main heterogeneity determinants.
Even the most optimistic estimates show that this urine collection technique is unreliable. The fact that 1 out of every 2 urine cultures collected with a perineal bag is contaminated or gives a false positive compromises the usefulness of the technique, or should at least make us question the validity of its results.
The high percentages of contamination and false positives are related to the collection procedure, i.e., cleanliness, placement and replacement of the perineal bag. The risk of contamination varies depending on the meticulousness of the procedure followed.10 Based on our results, the risk of urine contamination in children is somewhat higher, although it is unacceptably high in both cases. Although the percentage of false positives is lower in the studies in which the samples do not include an over-representation of patients with prior positive urine cultures, it is, in any case, very high. These studies were not excluded from the meta-analysis because it is common for new samples for culture to be collected from children with prior positive urine cultures (with bag). This tends to overestimate the positive cases in the samples, but it should not affect the confirmation percentage (false positives) of reference techniques.
It is surprising to observe that the 5 studies assessing the validity of sterile collection techniques found them to have a sensitivity and specificity of over 80%. It is evident that the exclusion of contaminated samples (which are neither positive nor negative, and are common in clinical practice) from the validity analysis would bias the estimation. Nevertheless, we cannot rule out the existence of certain settings and patient populations in which these results would be valid if strict hygiene measures are enforced.
The results of this review show the limitations of the studies included. Most of them are of medium quality, since none include such an important criterion as the blind or independent assessment of the diagnostic test and the reference criterion. In addition, the patient samples included are not sufficiently representative due to bias in the inclusion of samples previously positive or contaminated. Likewise, it is surprising that most of the studies are carried out in the hospital environment, although many of the children with UTI symptoms are seen at primary care clinics.
Although different documents and clinical practice guidelines already recommend the use of other urine collection techniques when a reliable diagnosis is essential,1,2 the findings of this review quantify the importance and significance of this recommendation. Considering the high risk of contamination, the perineal bag cannot be used to collect a sample for culture in a high-risk patient requiring immediate antibiotic treatment. A finding of contaminated urine will not confirm or rule out infection, and a positive finding runs a high risk of being false. Also, the antibiotic administered would interfere with the validity of the new urine sample that would have to be collected to confirm diagnosis. The diagnostic uncertainty would affect not only treatment but also the decision to perform further diagnostic tests or follow-up studies. The iatrogenic cascade triggered by each wrong diagnosis must also be considered.
In low-risk patients, in whom diagnosis is unlikely and treatment delay acceptable, we could collect the urine with a bag and wait for the result. The less invasive character and ease of this technique have made it the gold standard in our clinical practice. However, it is important to bear in mind that only negative results will be acceptable because the positive ones will most likely be false and will have to be confirmed with more valid urine collection techniques (bladder catheterisation or suprapubic aspiration). This recommendation, included in consensus documents,1,2 is pragmatic, even assuming that meticulous cleaning, placement and replacement of the bag will minimise the drawbacks of this urine collection technique.
We conclude that the perineal adhesive bag is not a sufficiently valid method for collecting urine for culture, since almost half of the cultures will be contaminated and 2 out of 3 positives will be false. Even though the estimates are inaccurate, due to the heterogeneity of the studies available, they must be considered when choosing the urine collection method. The sensitivity and specificity estimates are not applicable since they do not take into account the risk of contamination.
Conflict of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Ochoa Sangrador C, Pascual Terrazas A. Revisión sistemática de la validez de los urocultivos recogidos con bolsa estéril perineal. An Pediatr (Barc). 2016;84:97–105.