The term “sweetener” refers to a food additive that imparts a sweet flavour and usually provides no or very low energy. It is used to sweeten foods, medicines and food supplements with no nutritional purposes. For years, no-calorie sweeteners have been used as substitutes for all or part of the sugar content in foods and beverages. In recent decades its consumption has risen to prevent tooth decay, or as an aid in weight control, obesity and diabetes and, in general, to achieve an optimal energy balance. However, consumption of sugary or sweetened food and soft drinks is high, making this situation of special interest in calorie intake and in the poor behavioural pattern of eating habits in children. In addition, questions remain among consumers about the risks to health associated with their use, whether they are artificial or natural. The “artificial sweeteners” are the group of greatest interest in research in order to demonstrate their safety and to provide firm data on their possible therapeutic effects. The aim of the present document is to increase information for paediatricians on the characteristics of different sweeteners, and to advise on the choice of sweeteners, based on their properties.
El término edulcorante hace referencia a aquel aditivo alimentario que confiere un sabor dulce y que, habitualmente, no aporta o proporciona muy poca energía. Se utiliza para endulzar alimentos, medicamentos y complementos alimenticios cuando se persiguen fines no nutritivos. Desde hace años, se han empleado edulcorantes acalóricos como sustitutos de todo o parte del contenido en azúcares en comidas y bebidas. En las últimas décadas, se ha incrementado su consumo para prevenir la caries y para el correcto cumplimiento de la dieta en casos de control del peso corporal, obesidad y diabetes y, en general, como coadyuvantes para conseguir un balance energético adecuado. No obstante, el consumo de alimentos y de bebidas azucaradas y/o edulcoradas es elevado, reflejando o un aporte calórico importante, o un patrón de hábitos alimentarios inadecuados en los niños. Por otro lado, sigue habiendo dudas entre los consumidores sobre los riesgos para la salud asociados al uso de edulcorantes, ya sean artificiales o naturales. El principal interés en investigación sobre seguridad y los posibles usos terapéuticos se centra en los «edulcorantes artificiales». El objetivo de este documento es proporcionar información a los pediatras sobre las características de los distintos edulcorantes para aconsejar en la elección de un determinado edulcorante sobre la base de sus propiedades.
Carbohydrates (CHs) are the nutrients that constitute the main source of food energy. They are notable for their structure and pleasant taste, which in some cases, such as sugars, is sweet, making other foods more palatable, for their ability to satiate the appetite and, in some, for their high fibre content.1 CHs should provide between 45% and 60% of total dietary energy intake in children older than one year of age.2 CHs from food are presented in the form of complex molecules (polymers or polysaccharides), especially starches, dextrins and fibre, or simpler ones, commonly called sugars. The main dietary sources of sugars are fruit and fruit juices, some vegetables, milk, and processed foods with added sugars, especially sucrose or hydrolysed starch (glucose or fructose syrups), such as soft drinks, pastries, sweets and confectionery.1,2 Sugars are used to sweeten or enhance the flavour of many of them, to modify the freezing and melting point or to colour foods naturally, and to preserve them. Balanced intake of sugars in the daily diet has important properties, as it facilitates the rapid supply of glucose, an indispensable carbohydrate for the development of cognitive functions and physical activity, to the brain and muscles. Sugar should be consumed in a natural form with the foods that contain it, since this also provides other micronutrients. In the twentieth century, however, questions began to be raised as to whether excessive consumption of sugars, particularly associated with processed foods, might be related to diabetes or obesity, and research has continued up to the present.3–5
Additives are substances deliberately added to foods to perform certain technological functions and the result is that both the additive itself and its byproducts become components of those foods. Additives are not consumed as foods or used as typical ingredients in the diet, regardless of whether or not they have nutritional value. Monosaccharides, disaccharides or oligosaccharides, or foods containing them, are not regarded as food additives. The term sweetener refers to a substance used to impart a sweet taste to foods (Regulation (EC) No 1333/2008). Thus foods such as honey or ordinary sugar, fructose or glucose are not considered sweeteners, since they have other functions apart from sweetening.6
Low- or no-calorie sweeteners (LNCSs) have been used for years to replace all or part of the sugar content in foods and drinks, but in the last few decades their consumption has increased both in adults and in children. Their use is linked to dietary alternatives for weight control or diabetes, but also to preventing tooth decay. Although some studies question these possible benefits, both in adults and in children,7,8 systematic reviews and meta-analyses on this subject conclude that the use of sweeteners is beneficial in weight-control and diabetes programmes associated with a healthy lifestyle.9
The purpose of this document is to provide paediatricians with information on sweeteners and health-related issues, in order to give appropriate advice to patients and their families.
Consumption of sugars and sweeteners and its relationship with healthTo assess this consumption we have to take into account not only added sugars and sweeteners, but also sugar incorporated as an ingredient in precooked/processed foods. European adolescents consume some 384kcal per day from drinks, of which 30.4%, 20.7% and 18.1% comes from sugar-sweetened beverages, sweetened milk and fruit juice, respectively.10 Various cross-sectional studies have concluded that there is no association, or even that there is a negative association, between consumption of sugars and weight gain.11,12 However, there is a widespread debate on whether greater intake of sugars through sweetened beverages could have a significant effect on increase in body mass index (BMI) or about whether unsweetened diets may have an influence on control of obesity.13
Some observational studies have also related increase in BMI to consumption of non-caloric sweeteners, although these data should not be interpreted as proof of a causal relationship, but rather as a sign that the probability of consumption is higher in the obese and sedentary population (reverse causality).8 Replacing sugar with sweeteners is not necessarily associated with a lower overall calorie intake, and could encourage unbalanced dietary habits, involving high consumption of low-calorie products with sweeteners and of others with excessive calories.6,14 Some authors postulate that early consumption of sugar-sweetened products in infants and young children could affect self-regulation of eating and preference for sweet flavours,7,15 which may be maintained during childhood and adolescence. It has even been suggested that consuming sweeteners and sugars together in the diet could give rise to a neuronal response that leads to more rapid absorption of sugars, also increasing secretion of peptides related to glucagon, or insulin.16 On this subject a highly controversial hypothesis has recently been put forward on the development of glucose intolerance through alterations to the intestinal microbiota in mice associated with consuming saccharin at maximum doses.17 However, one must be very cautious in extrapolating conclusions from animal studies to humans.18 Nowadays diabetic patients can use non-caloric sweeteners as part of a balanced, controlled diet, and studies in adults indicate that they do not affect plasma glucose or lipid levels, although this has not been sufficiently investigated in children.19,20
Fermentable CHs in the diet produce acidic materials which destroy hard tissues of the tooth, giving rise to tooth decay. In the child population dental caries is still common, because of a general lack of adequate oral hygiene after consuming sugary foods, as well as fluoride deficiency.21 In infants, moreover, prolonged use of fruit juices or other fructose- or sucrose-rich sweetened beverages in dummies increases the risk of damage to teeth. Among sweeteners, polyols (sugar alcohols) and sucralose, among others, have been declared to have healthy properties for avoiding this condition.22
Recommendations for consumptionAll types of CHs must be represented in a healthy diet. The FAO and WHO recommend an intake of sugars (simple carbohydrates) of less than 10% of the total caloric value of the diet, trying to make them part of a healthy diet with limited consumption of sugar-sweetened drinks.23 In 2005 it was established that the recommended daily intake in adults and children older than one year of age is 100g/day of CHs as an estimated mean requirement.24 In Spain, adhering to a Mediterranean or similar diet would make it possible to maintain an appropriate energy intake without sacrificing its sweetening and pleasurable function. In addition, as a practical measure to achieve greater benefits, it has been recommended that consumption of sugars or sugar-sweetened foods should be limited to less than 3 times per day, not exceeding 6% of total energy intake,25 and that of sugar-sweetened drinks to occasional use. It is also recommended that excessive consumption of high-fructose corn syrup (55% fructose) should be reduced, as it represents a health hazard, especially for children.26 Indeed, these syrups are not used in Spain, where the maximum fructose content of manufactured products is approximately 50%.
The nutrition statements prescribed in Regulation (EC) No 1924/2006 make it easy to ascertain the composition of foods in terms of sugars. Thus according to the labelling we can find foods that are “low in sugar” (no more than 5g of sugars per 100g for solids or 2.5g of sugars per 100ml for liquids), “sugar-free” (no more than 0.25g per 100g or 100ml), or “with no added sugar” (a product with no added mono- or disaccharides nor any other food used for its sweetening properties). In the last of these cases, the food may “contain naturally occurring sugars” or “sugar(s) and sweetener(s)”, and this information must appear on the nutrition information label.27 On a practical level, the American Heart Association has suggested simple advice, such as not having more than 6 teaspoonfuls of sugar per day for women (25g) (e.g., present in 250ml of a sugar-sweetened beverage) and 9 for men (37g).28
During pregnancy CH intake should not be restricted, but intake of refined sugar or foods with added sugar should be controlled. There is an increase in availability of glucose, which is an essential substrate for the foetus. In newborns, because of pancreatic amylase deficiency, it is advisable for infant milk formulae not to contain starch, with lactose as the main CH, as well as glucose polymers, which can also be added, since they are directly absorbable by the enterocyte. On the other hand, the infant's predisposition towards sweet taste makes it possible to introduce new foods with a relatively high starch content.29
As for sweeteners, the European Food Safety Authority (EFSA) proposes acceptable daily intakes to ensure appropriate use without possible adverse effects.30 In the case of infants it is difficult to find sweeteners in the products they consume, as they are prohibited in milk formulae, cereals and baby foods. At later ages, foods such as soft drinks, fruit juices or dairy products sweetened with sugar or sweeteners should be controlled. Reducing the energy content in some foods through sweeteners does actually seem to have modest benefits in children.16 On the other hand, it may lead to greater consumption of other more caloric foods, and there is also the possibility that early exposure to sweeteners, as mentioned previously, could affect dietary habits later.31 In pre-school and school-aged children the recommendations already described should be maintained, taking some additional factors into account, such as physical activity, which may mean that sugar intake has to be regulated with appropriate beverages rather than just drinking water.32 Indeed, anaerobic or prolonged exercise causes a depletion of liver and muscle glycogen reserves, leading to fatigue and cognitive abnormalities,33 which sometimes have to be mitigated with sugars.
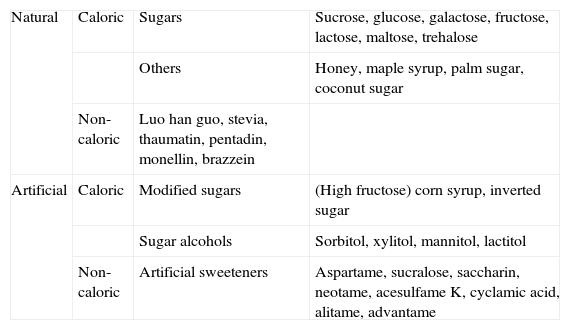
Types of sweetenersThere are various ways of classifying sweeteners (Tables 1 and 2). Using the glycaemic index (GI) we can also make a classification of foods based on postprandial blood sugar response, comparing them with a reference food (GI=100). Sucrose is medium GI (≈65).34 Depending on their energy contribution they are classified as “caloric” or “low calorie/non-caloric” (Table 1) or as “nutritive” or “non-nutritive”, as established by the US Food and Drug Administration (FDA) (Table 2).35 In some cases the term “natural origin” is used in the consumer information, as with steviol glycoside, or “artificial” origin when they are synthetic (Table 1).
Classification of sugars and sweeteners.
| Natural | Caloric | Sugars | Sucrose, glucose, galactose, fructose, lactose, maltose, trehalose |
| Others | Honey, maple syrup, palm sugar, coconut sugar | ||
| Non-caloric | Luo han guo, stevia, thaumatin, pentadin, monellin, brazzein | ||
| Artificial | Caloric | Modified sugars | (High fructose) corn syrup, inverted sugar |
| Sugar alcohols | Sorbitol, xylitol, mannitol, lactitol | ||
| Non-caloric | Artificial sweeteners | Aspartame, sucralose, saccharin, neotame, acesulfame K, cyclamic acid, alitame, advantame |
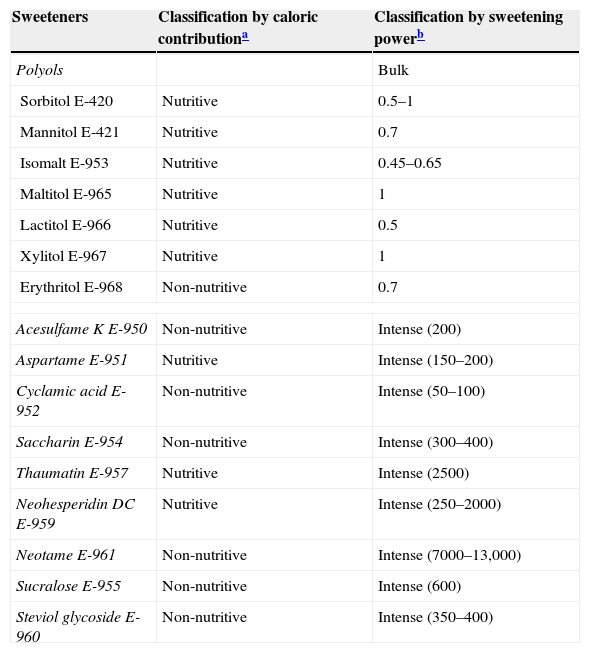
Classification of sweeteners according to the US Food and Drug Administration (FDA) and the European Union, and the sweetening power (SP) of each substance relative to sucrose (table sugar): degree of sweetness comparable with sugar (bulk sweetener) or much higher (intense sweetener).
| Sweeteners | Classification by caloric contributiona | Classification by sweetening powerb |
|---|---|---|
| Polyols | Bulk | |
| Sorbitol E-420 | Nutritive | 0.5–1 |
| Mannitol E-421 | Nutritive | 0.7 |
| Isomalt E-953 | Nutritive | 0.45–0.65 |
| Maltitol E-965 | Nutritive | 1 |
| Lactitol E-966 | Nutritive | 0.5 |
| Xylitol E-967 | Nutritive | 1 |
| Erythritol E-968 | Non-nutritive | 0.7 |
| Acesulfame K E-950 | Non-nutritive | Intense (200) |
| Aspartame E-951 | Nutritive | Intense (150–200) |
| Cyclamic acid E-952 | Non-nutritive | Intense (50–100) |
| Saccharin E-954 | Non-nutritive | Intense (300–400) |
| Thaumatin E-957 | Nutritive | Intense (2500) |
| Neohesperidin DC E-959 | Nutritive | Intense (250–2000) |
| Neotame E-961 | Non-nutritive | Intense (7000–13,000) |
| Sucralose E-955 | Non-nutritive | Intense (600) |
| Steviol glycoside E-960 | Non-nutritive | Intense (350–400) |
Natural caloric sweeteners include sucrose, fructose, glucose and maltose. Fructose has classically been used as a substitute for sucrose in diabetic patients. More recently, however, it has been found that high-fructose diets, especially if the fructose is added to processed foods, could induce hyperinsulinaemia, hypertriglyceridaemia and insulin resistance, and this has led to a recommendation to limit their use in diabetics. In addition, fructose dependence has been described, leading to high consumption.36
Fructooligosaccharides have a sweetening power relative to sucrose of 0.3–0.6. Inulin is a fructan with a well-known prebiotic effect found naturally in the yacon or Peruvian ground apple, a tuber native to the Andes, considered a health food on account of being rich in various minerals, vitamin C and B-group vitamins. Coconut sugar is another traditional product with a low GI because of its sucrose content. Polyols derived from sugar and also regarded as CHs are produced, albeit in small quantities, in plants and cereals. They generally contain fewer calories than sugar, with a very low GI, and have not been associated with the development of tooth decay, since they are non-fermentable; they are used as sweeteners. Their intestinal absorption is generally low, and in moderate quantities (more than 10g of sorbitol) they can produce flatulence, colic or diarrhoea, as they are fermented by the colonic microbiota.
Industrial processed foods use sugars converted from starch, with a high GI and high calorie content. Corn syrup is the prime example.
There are also natural sweeteners (stevia, luo han guo, thaumatin and brazzein) with no significant calorie content in the quantities normally consumed for sweetening purposes. These are not CHs, and therefore have no GI. They are regarded as high-intense sweeteners.
“Artificial sweeteners” are characterised by being non-caloric, with no glycaemic effect and high sweetening power (Table 2). Prominent among them is saccharin, which has great sweetening capacity. Sucralose and aspartame are also notable for their extensive worldwide use, especially in beverages.37 Aspartame is made from a methyl ester of phenylalanine and aspartic acid. Its use is approved by the FDA and the EFSA.38
Safe use of sweetenersSociety demands a market supply of high-quality food substances that are suitable for consumption by people with specific needs, such as diabetics, or that respond to the current demand for low-calorie products. Although the object of using LNCSs is to reduce calorie intake in the diet and the presence of fermentable sugars in the mouth, seeking beneficial effects, such as promoting a decrease in body weight, and preventing diabetes or tooth decay, consumers have doubts about the health risks associated with their use, depending on whether they are artificial or natural. The fact that a sweetener is of natural origin does not imply greater safety or effectiveness. Since a wide range of molecules is involved, there are many potential sources of risk: interference in absorption, metabolism or excretion of nutrients or intermediate metabolites, as well as allergic reactions, accumulation in tissues, effects on normal intestinal flora, disruption of blood glucose regulation or interaction with other drugs or medications.
In research on safety and possible therapeutic uses in patients with diabetes or other specific health problems the focus of interest is on “artificial sweeteners”. For this purpose the legal aspects of safety and efficacy in the use of sweeteners need to be constantly reviewed by the EFSA.
Regulation (EC) No 1333/2008 lays down rules on the food additives used in foods to ensure the protection of consumers’ health while upholding trade practices. Annex II, updated in 2013, indicates that the following LNCSs are authorised: acesulfame-K (E-950), aspartame (E-951), salt of aspartame-acesulfame (E-962), cyclamic acid and its Na and Ca salts (E-952), neohesperidine DC (E-959), saccharin and its Na, K and Ca salts (E-954), sucralose (E-955), thaumatin (E-957) and neotame (E-961). Following the favourable verdict from the EFSA in 2010, the use of stevia derivatives, steviol glycosides (E-960), as natural non-caloric sweeteners was definitively approved throughout the European market, and also sorbitol and xylitol. In 2014 the use of advantame (E-969) as an intense sweetener was authorised.
With regard to aspartame, it does not represent a risk of toxicity for consumers at current exposure levels. Because it is a source of phenylalanine it is not recommended in individuals with hyperphenylalaninaemia or phenylketonuria, although in studies in patients with consumption at normal doses and long-term studies, even in pregnant women, no adverse effects have been found.37
Information for correct use of these substances is based on knowing the differences between the information labels of commonly consumed products containing sweeteners. The labelling of food additives must comply with the general conditions laid down in Directive 2000/13/EC. Specifically, if the product contains polyols, the label must state: “excessive consumption may produce laxative effects”, or if it contains aspartame or salt of aspartame-acesulfame: “contains a source of phenylalanine”. In this regard, however, there is substantial room for improvement to ensure that this compulsory information appears on all products containing sweeteners.
Under European regulations (Council Directive 89/398/EEC), artificial sweeteners must not be used in infant formulae, follow-on formulae, cereals, baby foods or dietary foods for very young children for special medical purposes, unless expressly indicated.38 Sugars such as sucrose or fructose may be added in limited quantities.
Scientific research, though still scarce in humans, shows that sweeteners are safe in the general population, including pregnant women39 and children, although in these populations they must be used in moderation.5,16,22
All the food additives approved in the European Union are considered safe in the specified doses and conditions of use. In children, however, additives of this kind should only be used as an alternative when other forms of obesity prevention are not sufficient, except for the use of sugar-free chewing gum to prevent tooth decay, or use in pharmaceutical products.5
Final comments- 1.
In general, an intake of simple CHs of less than 10% of the caloric value of the diet is appropriate in the context of a healthy lifestyle.
- 2.
Sweeteners, especially “non-caloric” sweeteners, can help to limit the intake of refined sugars in the diet and are useful for preventing diseases such as obesity and diabetes, in association with a moderate, balanced diet.
- 3.
In view of the recommendation not to add sweeteners to foods intended for infants and very young children, their use is not advisable in children between the ages of 1 and 3 years.
- 4.
It is recommended that health care professionals acquire the appropriate knowledge to advise on and/or choose a particular sweetener on the basis of its properties.
- 5.
There is a continuing need for specific research in order to make appropriate use of sweeteners in children.
The authors have no conflicts of interest to declare.
José Manuel Moreno Villares (coordinator), Juan José Díaz Martín, Mercedes Gil Campos, Ana Moráis López, Víctor Manuel Navas López, Susana Redecillas Ferreiro, Miguel Sáenz de Pipaón, Miguel Ángel San José González, Félix Sánchez-Valverde Visus.
The members of the Comité de Nutrición de la AEP are presented in Appendix A.
Please cite this article as: Gil-Campos M, San José González MA, Díaz Martín JJ. Uso de azúcares y edulcorantes en la alimentación del niño. Recomendaciones del Comité de Nutrición de la Asociación Española de Pediatría. An Pediatr (Barc). 2015;83:353.e1–353.e7.