To show the preparation process by the Poisoning Working Group of the Spanish Society of Paediatric Emergencies (GTI-SEUP), of the list of things “not to do” for a paediatric patient who has been exposed to a potentially toxic substance.
MethodThe preparation process of the list was carried out in three phases. First: “Brainstorming” that was open to all members of the GTI-SEUP. Second: Recommendations were selected by following modified-Delphi methodology. All participants were asked to rate the proposals (from 1 = strongly disagree to 9 = strongly agree). Those with an average score greater than 8 were accepted (provided that at least two-thirds of the participants had given them a score ≥ 7), and a second consultation was made for the recommendations with an average score between 6 and 8. Third: Writing and creating a consensus of the final document was done.
ResultA total of 11 proposals were initially obtained. Thirty-two of the 57 GTI-SEUP participants completed the scoring questionnaire. In the first consultation, seven “not to do” recommendations were accepted, and four obtained a doubtful average score (between 6 and 8). After the second consultation, the list was made up of eight recommendations. Two refer to general management, four to gastrointestinal decontamination techniques, and two to the administration of antidotes.
ConclusionThe list of actions that should not be taken with a child that has been exposed to a possible poison is a consensus tool, within the GTI-SEUP, to promote improvement in the quality of care offered to these patients. This improvement is based on avoiding unnecessary measures, which can sometimes be harmful to the child.
Mostrar el proceso de elaboración, dentro del Grupo de Trabajo de Intoxicaciones de la Sociedad Española de Urgencias de Pediatría (GTI-SEUP), de la lista de recomendaciones de “no hacer” ante un paciente pediátrico que ha contactado con una sustancia potencialmente tóxica.
MétodoEl proceso de elaboración de la lista se realizó en 3 fases. Primera: “Lluvia de ideas” abierta a todos los miembros del GTI-SEUP. Segunda: Selección de las recomendaciones, siguiendo una metodología Delphi-modificada. Se solicitó a todos los participantes que puntuasen las propuestas (del 1 = totalmente en desacuerdo al 9 = totalmente de acuerdo). Se aceptaron aquellas con una puntuación media superior a 8 (siempre que al menos 2/3 de los participantes le hubieran otorgado una puntuación ≥ 7) y se realizó una segunda consulta para las recomendaciones con una puntuación media entre 6 y 8. Tercera: Redacción y consenso del documento final.
ResultadoInicialmente se obtuvieron 11 propuestas. Treinta y dos de los 57 participantes del GTI-SEUP respondieron al cuestionario de puntuación. En la primera consulta, fueron aceptadas 7 recomendaciones de “no hacer” y 4 obtuvieron una puntuación media dudosa (entre 6 y 8). Tras la segunda consulta, la lista quedó formada por 8 recomendaciones. Dos hacen referencia al manejo general, 4 a técnicas de descontaminación digestiva y 2 a la administración de antídotos.
ConclusiónLa lista de acciones que no hay que hacer ante un niño que ha contactado con un posible tóxico es una herramienta consensuada, dentro del GTI-SEUP, para promover una mejora de la calidad asistencial ofrecida a estos pacientes. Dicha mejora se basa en evitar medidas innecesarias, que en ocasiones pueden resultar nocivas para el niño.
Paediatric poisonings are potentially serious events that are rare and widely heterogeneous and doubts frequently emerge as to how they should be managed. Previous studies have shown that the management of cases of poisoning in paediatric emergency departments (PEDs) in Spain could improve, especially as regards performance of gastrointestinal decontamination (GD).1,2
In 2010, the working group on poisoning (WGP) of the Sociedad Española de Urgencias de Pediatría (Spanish Society of Paediatric Emergency Medicine, SEUP) established quality indicators for the management of paediatric poisoning.3 These indicators have allowed monitoring of the use of GD methods, among other practices. Despite the implementation of some measures for improvement, the proportion of GDs that include gastric lavage has remained above the established target (<10%).4,5 There is also evidence that activated charcoal (AC) is frequently administered past the time frame when it could be effective.5,6 Since all GD methods may give rise to iatrogenic complications, they should only be used when indicated.7
Choosing wisely or “do not do” recommendations are guidelines disseminated by scientific societies with the aim of eradicating inappropriate approaches to clinical management. This strategy aims at reducing unnecessary health care costs, preventing iatrogenesis and facilitating doctor-patient communication in the decision-making process.8 The American Academy of Pediatrics and the Asociación Española de Pediatría (Spanish Association of Pediatrics) have published 10 and 5 “do not do” recommendations, respectively, but none of them apply to the management of poisoning.9,10 In the field of clinical toxicology, the American College of Medical Toxicology and the American Academy of Clinical Toxicology (AACT) published a list of the Ten Things Physicians and Patients Should Question in 2013 with a strong emphasis on heavy metals and alternative medicines.11 Since exposure to these toxins is rare in children, this list is not very useful in paediatric practice. In Spain, the Fundación Española de Toxicología Clínica (Spanish Foundation of Clinical Toxicology, FETOC) published a proposal in 2015 with 5 interventions that should be avoided in the management of patients with acute poisoning, including performance of gastric lavage in patients at risk of aspiration.12
The aim of this article was to describe the process of the development by the WGP-SEUP of a list of “do not do” recommendations for the management of patients exposed to potentially toxic substances.
MethodsThe development process was structured in 3 phases. The first phase (May to June 2017) consisted in gathering suggestions for “do not do” recommendations. To do so, the 57 members of the WGP-SEUP were invited to participate in a brainstorming effort through electronic mail. All participants were provided with existing models to serve as examples,11,12 and emphasis was placed on the submission of suggestions fitting the type of patients with suspected poisoning that are managed in PEDs in Spain.
The second phase (July–October 2017) was devoted to the selection of recommendations. We submitted a questionnaire that featured every suggestion obtained in the brainstorming phase to every member of the WGP-SEUP, along with directions for the evaluation, which followed the structure of a modified Delphi process. We directed participants to rate each recommendation on a scale from 1 to 9 (1 = strongly disagree; 9 = strongly agree), to provide a rationale for the rating and to give suggestions on how to improve the wording, if they deemed it necessary. We explained the approval criteria, which had been established based on the previous literature.13,14 Recommendations with a mean rating greater than 8 (as long as at least 2/3 of participants had given them a rating of at least 7) were approved, and those with a mean rating of less than 6 were rejected. We carried out a second round in which we requested that participants reassess recommendations with a rating in the uncertainty range (6–8). In this second round, we provided the comments given in the previous round to justify the ratings, preserving the anonymity of the raters. The same approval and rejection criteria based on the mean score applied.
The third phase (November 2017) involved the writing and approval by consensus of the final document. This was done taking into account suggestions to improve the wording and, in case of disagreement, we asked the opinion of all participants one more time and finally selected the version that received the most votes. Once the final document was approved by consensus, we proceeded to design the layout and to seek the endorsement of scientific societies.
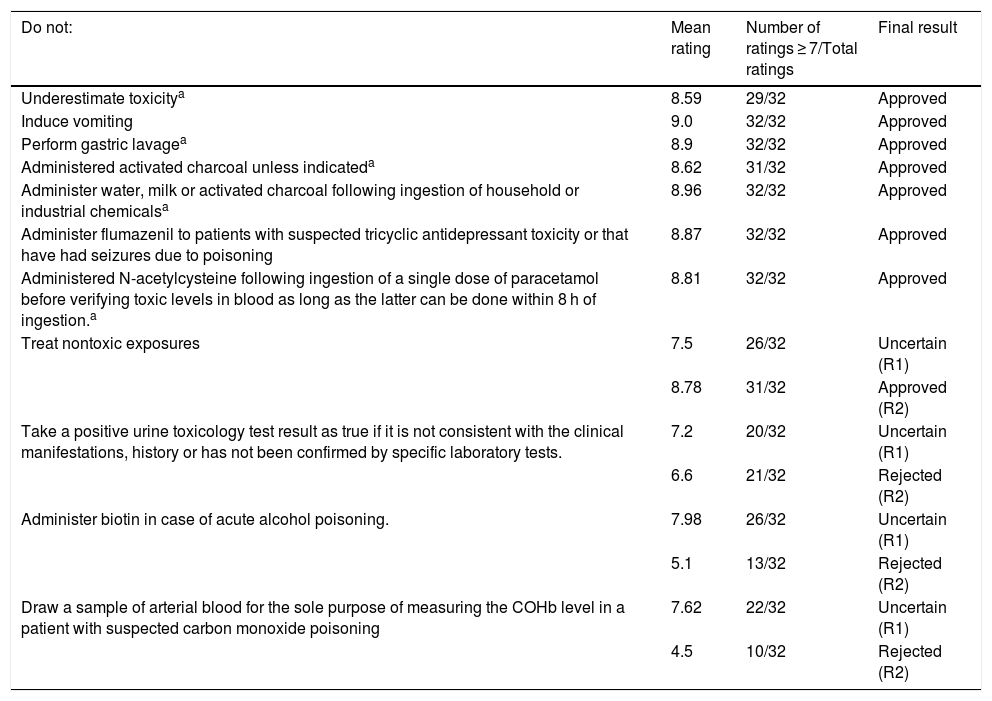
ResultsIn the brainstorming phase, we received 11 suggestions for “do not do” recommendations. A total of 32 paediatricians from different PEDs responded to the rating questionnaire. In the first round, 7 recommendations were approved, and 4 received a rating in the uncertain range (6–8 points). An eighth recommendation was approved in the second round. Table 1 presents the final ratings given to the suggested recommendations. The final draft was approved by consensus, followed by the layout of the document. We obtained the scientific endorsement of the FETOC and the SEUP. Fig. 1 shows the final list of recommendations, comprising 8 “do not do” interventions in the management of paediatric patients exposed to potentially toxic substances. Two recommendations refer to the management of suspected poisoning overall, 4 to GD interventions and 2 to the administration of antidotes.
Ratings given to the proposed “do not do” interventions in the management of paediatric patients exposed to potentially toxic substances.
| Do not: | Mean rating | Number of ratings ≥ 7/Total ratings | Final result |
|---|---|---|---|
| Underestimate toxicitya | 8.59 | 29/32 | Approved |
| Induce vomiting | 9.0 | 32/32 | Approved |
| Perform gastric lavagea | 8.9 | 32/32 | Approved |
| Administered activated charcoal unless indicateda | 8.62 | 31/32 | Approved |
| Administer water, milk or activated charcoal following ingestion of household or industrial chemicalsa | 8.96 | 32/32 | Approved |
| Administer flumazenil to patients with suspected tricyclic antidepressant toxicity or that have had seizures due to poisoning | 8.87 | 32/32 | Approved |
| Administered N-acetylcysteine following ingestion of a single dose of paracetamol before verifying toxic levels in blood as long as the latter can be done within 8 h of ingestion.a | 8.81 | 32/32 | Approved |
| Treat nontoxic exposures | 7.5 | 26/32 | Uncertain (R1) |
| 8.78 | 31/32 | Approved (R2) | |
| Take a positive urine toxicology test result as true if it is not consistent with the clinical manifestations, history or has not been confirmed by specific laboratory tests. | 7.2 | 20/32 | Uncertain (R1) |
| 6.6 | 21/32 | Rejected (R2) | |
| Administer biotin in case of acute alcohol poisoning. | 7.98 | 26/32 | Uncertain (R1) |
| 5.1 | 13/32 | Rejected (R2) | |
| Draw a sample of arterial blood for the sole purpose of measuring the COHb level in a patient with suspected carbon monoxide poisoning | 7.62 | 22/32 | Uncertain (R1) |
| 4.5 | 10/32 | Rejected (R2) |
Based on the “do no harm” principle, the WGP-SEUP developed a list of “do not do” recommendations for the management of paediatric patients exposed to potentially toxic substances using a Delphi approach. Delphi approaches have proven useful for the selection of quality indicators and Choosing wisely recommendations in the past.13–16
The Choosing wisely campaign was originally launched in the United States with the aim of reducing unnecessary health care expenditure, believed to amount to up to 30% of total health care costs.17 It focused on commonly used procedures and treatments shown by the existing literature to be overutilized. Paediatric visits due to exposure to toxic substances, which is much less frequent compared to other presenting complaints in the PED setting and account for an almost negligible percentage of the total health care expenditure, have not been addressed in any of such recommendations made to date.18
With the creation of the “do not do” recommendation list, the WGP-SEUP continues the work it has been developing in the past 20 years to establish the characteristics of paediatric poisoning cases in Spain and to improve the quality of care provided to paediatric patients with poisoning. The main goal of these recommendations is to reduce the use of interventions that may be iatrogenic, with particular emphasis on GD practices.
At the beginning of the 21st century, it was not an uncommon practice in PEDs to start the care of a patient that had ingested a potentially toxic substance with “why don’t you start the gastric lavage and I’ll be around soon to see them”. At present, the “8 not-to-dos” remind us that routine performance of GD should be avoided and that patient care should be personalized and adapted to the risk involved in each given situation. The care of patients with potential poisoning must start with an anamnesis and physical examination, stabilization, if needed, and assessment of toxicological risk.19,20 If the evaluation concludes that there is no risk of poisoning, the management should be limited to interventions aimed at reducing the risk of future events by providing recommendations for prevention to the family.21
The position statements on the use of GD methods of the AACT and the European Association of Poisons Centres and Clinical Toxicologists published in 1997 and successive updates conclude that the use of emetics should be avoided22–24 and that gastric lavage should only be used in exceptional cases.25–27 Thus, the most recent update states that “in the rare situation where gastric lavage might seem appropriate, clinicians should consider treatment with activated charcoal or observation and supportive care in place of gastric lavage” and emphasises that gastric lavage should only be performed by individuals with proper training and expertise.27 This very strict statement is based on the evidence supporting the use of gastric lavage for the few established indications (lethal ingestion, recent exposure, substance not bound to AC) being weak and based on theoretical grounds or case reports. In contrast, there is solid evidence on the risks associated with the use of gastric lavage.27
When it comes to activated charcoal, studies in healthy volunteers have shown that immediate administration significantly reduces the absorption of most drugs. However, this beneficial effect is very time-dependent and disappears after 2 h from ingestion except for extended-release drugs and medications that delay gastric emptying.28,29 There is also evidence that AC does not adsorb iron or lithium salts, among others, and that the risk of complications contraindicates its use in the absence of an intact or secured airway or after ingestion of hydrocarbons. Its use is also not indicated to treat ingestion of caustic substances, as it cannot adsorb them, it would interfere in the endoscopic assessment of the gastrointestinal mucosa and would increase the risk of injury in case of vomiting.29
The administration of any liquid or solid is generally considered contraindicated if the development of vomiting could increase the risk of aspiration (for instance in cases of hydrocarbon ingestion)30 or gastrointestinal mucosa injury (caustic substances).31 Therefore, and even if a thorough evaluation of the case thereafter alters the applicability of this recommendation, administration of water, foods or AC in cases of ingestion of household or industrial chemical products is not recommended in the initial management.
Another of the pillars of poison-specific treatment is the administration of antidotes. However, all antidotes pose a risk of adverse events, so the indication for their use must be considered with care. Flumazenil is one of the antidotes with the least favourable risk-benefit ratio. On one hand, benzodiazepine toxicity is usually benign, but on the other, the risk of triggering seizures in susceptible patients (ingestion of substances with proconvulsant effects or previous history of seizures) is a contraindication, either absolute32,33 or relative,34 to the use of benzodiazepines in these patients. Tricyclic antidepressant poisoning poses a particularly high risk in which the acidosis caused by convulsions can promote the development of malignant arrhythmias.32
Anaphylactoid reactions are a well-documented potential adverse effect of treatment with N-acetylcysteine (NAC) in patients with paracetamol poisoning. Yarema et al. reported an incidence of 8.2% in a sample of nearly 6500 patients treated with the standard 21-h protocol. They also found that female patients, patients that ingested a single dose and patients with lower serum levels of paracetamol were at higher risk.35 Given the considerable efficacy of NAC when it is administered within 8 h of ingestion, the main guidelines recommend its use in the context of a single ingestion of paracetamol at a potentially toxic dose, if the serum concentration of the drug is above the Rumack-Matthew nomogram line or if the concentration cannot be determined in the first 8 h following ingestion.36,37 As an exception, massive paracetamol ingestions indicate the immediate administration of N-acetylcysteine.
The list of “not to do” interventions of the WGP-SEUP also intends to raise awareness on other aspects that may result in harm to patients, such as underestimated risks. Recently, an extensive review was published that analysed the evidence on the “one-pill killers” or drugs that may be lethal with ingestion of one or a few units available in Spain.38 This article may be useful to facilitate the identification by paediatricians and general physicians of small-volume ingestions the risk of which may be underestimated.
The main limitation of this work is that the list of “eight not-to-do interventions” has been elaborated by expert consensus. To alleviate this limitation, the WGP-SEUP based these recommendations on the most frequently encountered situations in PEDs for which there is the greatest volume of evidence, such as GD methods and the administration of the most common antidotes. It also used a consensus process that is supported by previous evidence.13–16
Another important limitation is that this “do not do” recommendation list does not offer any strategies to reduce inappropriate practices. We believe that the mere diffusion of these recommendations can contribute to achieving this goal, but it is still clearly necessary to make a detailed analysis of the management of children with suspected poisoning in Spanish PEDs to identify opportunities for improvement and propose specific strategies to pursue them. There is evidence that the use of quality indicators in the management of paediatric poisoning is beneficial to this end.2,5,6 In some cases, it may be necessary to develop new indicators based on the “do not do” interventions, as has been done with the Choosing wisely recommendations of the Society of Hospital Medicine Pediatric Committee.39
In conclusion, the list of “do not do” interventions in the management of a child exposed to a potentially toxic substance is a tool developed by the WGP-SEUP through a consensus process of demonstrated effectiveness to help improve the quality of the care provided to these patients. This improvement would be based in reducing the use of unnecessary interventions that might cause harm to the patient.
FundingThe authors have no sources of funding to disclose in relation to the present article.
Conflicts of interestThe authors have no conflicts of interest to declare.
A. Alday (Hospital Gernika-Lumo), A.G. Andrés (Complejo Asistencial Universitario de León), C.M. Angelats (Hospital Francesc de Borja de Gandía), E. Aquino (Hospital Virgen de la Salud), J. Astete (Fundació Sant Hospital de la Seu d´Urgell), I. Baena (Corporación Sanitaria Parc Taulí, Sabadell), A. Barasoain (Hospital Universitario Fundación Alcorcón), P. Bello (Hospital Rey Juan Carlos), C. Benito (Hospital Universitario Puerta del Hierro, Majadahonda), H. Benito (Hospital Universitario Rio Ortega), E. Botifoll (Hospital Sant Joan de Déu, Xarxa Hospitalària i Universitària de Manresa. Fundació Althaia), B. Burguera (Hospital Universitario Doctor Peset, Valencia), C. Campos (Hospital Miguel Servet), V. Canduela (Hospital de Laredo), N. Clerigué (Complejo Hospitalario de Navarra), C. Comalrena (Corporación Sanitaria Parc Taulí, Sabadell), T. Del Campo (Complejo Hospitalario de Jaén), B. De Miguel (Hospital Infantil La Paz), R. Fernández (Hospital de Cabueñes), B. Fernández (Hospital de Cabueñes), E. García (Hospital Nuestra Señora de Sonsoles de Ávila), M. García (Hospital Universitario Cruces), M. García (Hospital Universitario Infanta Elena), M.A. García (Hospital Príncipe de Asturias, Alcalá de Henares), C. García-Vao (Hospital Universitario del Tajo), L. Herrero (Hospital de Mendaro), P. Huerta (Hospital Clínico Universitario Lozano Blesa), J. Humayor (Hospital Universitario Basurto), P. Hurtado (Hospital Materno Infantil de Badajoz), I. Iturralde (Hospital Quirón, Bizkaia), A. Jordá (Hospital de Laredo), P. Khodayar (Hospital Clínico Universitario de Valencia), M. Lalinde (Hospital Montepríncipe; Hospital Sanchinarro; Hospital Torrelodones; Hospital Universitario HM Puerta del Sur), Z. Lobato (Hospital Sant Joan de Déu, Xarxa Hospitalària i Universitària de Manresa. Fundació Althaia), J. López (Hospital Universitario de Salamanca), V. López (Hospital Universitari Son Espases de Palma de Mallorca), C. Luaces (Hospital Sant Joan de Déu Barcelona), L. Mangione (Hospital Materno Infantil de las Palmas de Gran Canaria), L. Martín (Hospital Universitario Carlos Haya), L. Martínez S. (Hospital Sant Joan de Déu Barcelona), L. Martínez (Hospital San Pedro), J. Martorell (Hospital de Mataró), M.E. May (Hospital Universitario Mutua Terrassa), M.C. Melguizo (Hospital Universitario San Agustín, Linares), S. Mesa (Hospital Universitario Doce de Octubre), J.C. Molina (Hospital Universitario Niño Jesús), M. Muñiz (Complejo Asistencial Universitario de León), J.A. Muñoz (Hospital Universitario Donostia), N. Muñoz (Hospital Santa Bárbara de Soria), S. Oliva (Hospital Universitario Carlos Haya), M. Palacios (Complejo Hospitalario de Navarra), A. Pérez (Hospital de Zumárraga), C. Pérez (Hospital Clínico Universitario Virgen de la Arrixaca), M. Pinyot (Hospital de Terrassa), A. Peñalba (Hospital Universitario Marqués de Valdecilla; Hospital Sierrallana), N. Pociello (Hospital Universitario Arnau de Vilanova), A. Rodríguez (Hospital Universitario Doctor Peset, Valencia), M.D. Rodríguez (Hospital Infanta Cristina, Parla), R. Señer (Hospital Universitario y Politécnico la Fe), I. Serrano (Hospital Universitario Cruces), P. Vázquez (Hospital Universitario Gregorio Marañón), C. Vidal (Hospital Son Llatzer).
Appendix A lists the members of the Working Group on Poisonings of the Sociedad Española de Urgencias de Pediatría.
Please cite this article as: Martínez-Sánchez L, López-Ávila J, Barasoain-Millán A, Angelats-Romero CM, Azkunaga-Santibañez B, Molina-Cabañero JC. Acciones que no hay que hacer ante un paciente que ha contactado con un posible tóxico. An Pediatr (Barc). 2021;94:285–292.
Previous presentations: This study was presented at the 23 Meeting of the Sociedad Española de Urgencias de Pediatría, held in April 2008 in Sitges, Spain (award to the best communication by a working group of the SEUP/RiSEUP) and the 39 Congress of the European Association of Poison Centres and Clinical Toxicologists, held in May 2019 in Naples, Italy.