Hypertension (HTN) is an important cardiovascular risk factor in patients with type 1 diabetes (T1D), who are at increased risk the greater the duration and severity of HTN.1,2 For this reason, patients with T1D used to be screened for HTN by means of office blood pressure (BP) measurement, with use of ambulatory blood pressure monitoring (ABPM) to confirm the diagnosis. The most recent European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents recommend ABPM in patients with a T1D diagnosis,3 although due to its lower availability it is not used routinely.
The aim of our study was to describe the different BP phenotypes based on the isolated office BP measurements (clinic BP) and ABPM in children and adolescents with T1D and analyse the presence of differences in epidemiological, clinical or laboratory characteristics associated with high ambulatory BP values. We conducted a cross-sectional study in a cohort of children and adolescents with T1D followed up in the childhood diabetes unit of a tertiary care hospital. We included patients aged 5 to 18 years with T1D of more than 6 months’ duration without a prior history of HTN. The primary variables were the clinic BP and the 24-h ambulatory BP. We collected data on previous capillary glycated haemoglobin and lipid profile values as well as the body mass index (BMI) z-score relative to the reference population of the same age and sex.
We categorised clinic BP measurements as normal BP (systolic BP [SBP] and diastolic BP [DBP] < 90th percentile [P90]), high BP (SBP or DBP ≥ P90 and <95th percentile [P95]) or HTN (SBP or DBP ≥ P95 and4 At the office, BP was measured with a properly calibrated oscillometer. In patients with SBP and DBP values repeatedly above the P90, BP was measured manually with a sphygmomanometer and stethoscope.
Ambulatory blood pressure monitoring was performed with the Spacelabs 90207 monitor (Spacelabs Healthcare). All recordings contained at least 75% of valid readings. We defined HTN as a mean daytime, night-time and 24 h SBP or DBP above the 95th percentile (Ambulatory blood pressure monitoring in children and adolescents. Hypertension, 2008). We analysed the mean of the variables under study based on whether the ABPM was normal or pathological.
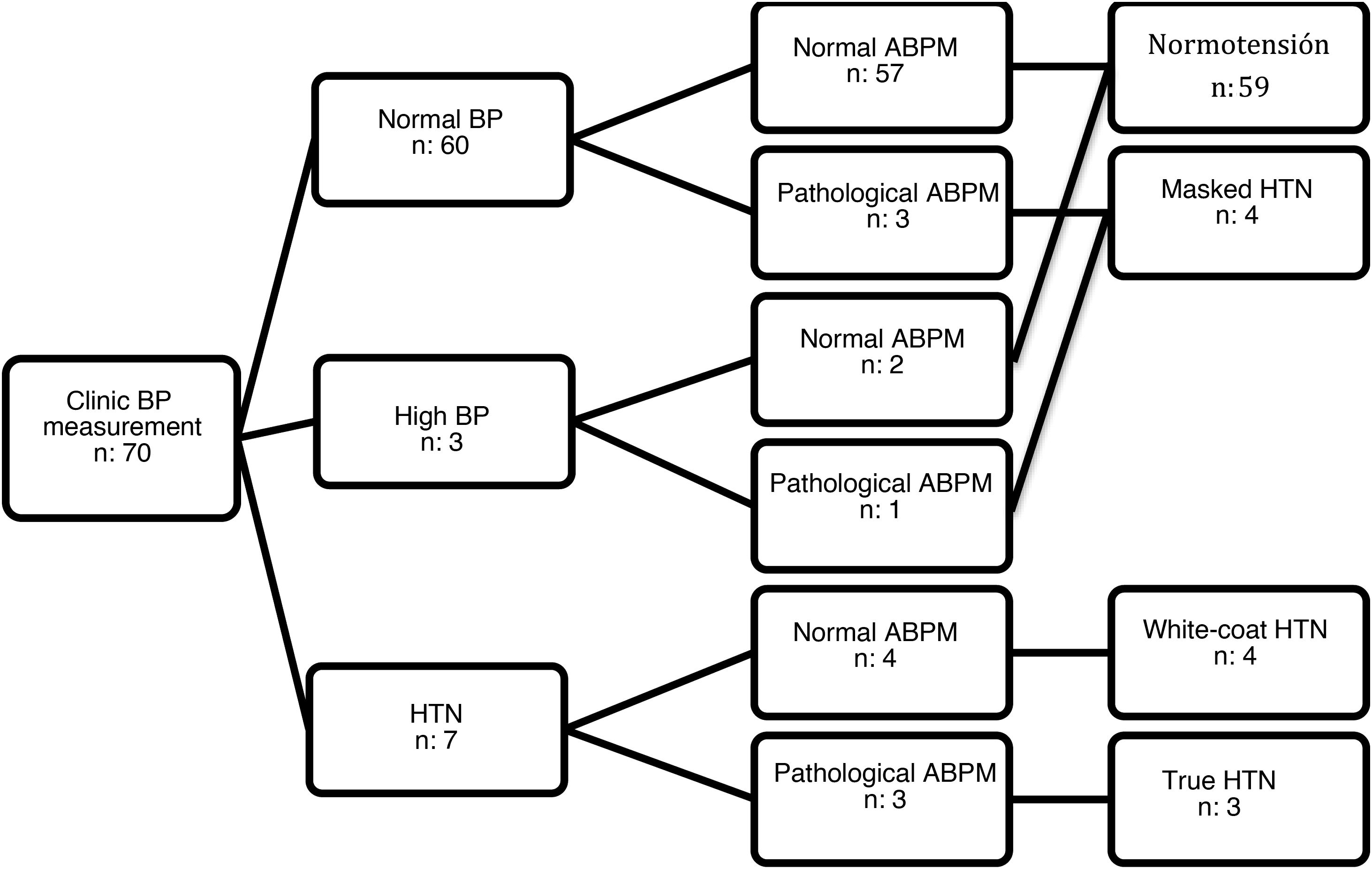
Seventy patients met the inclusion criteria (51.40% female). All agreed to participate in the study, and we obtained signed informed consent to participation. The mean age was 11.26 years (standard deviation, 2.67) and the median duration of T1D was 3.46 years (interquartile range, 4.10). The clinic BP was in the normal range in 60 patients, high in 3 and in the HTN range in 7. In the group of patients with normal/high BP, ABPM detected masked HTN in 4 patients. Of the 7 patients with clinic BP measurements in the HTN range, ABPM confirmed it in 3 (true HTN) and ruled it out in 4 (white-coat HTN) (Fig. 1). None of the patients with high ambulatory BP values had obesity (BMI z-score >2). Only 3 patients had a family history of HTN, without significant differences in family history between patients with HTN and patients with normal BP. Table 1 summarizes the characteristics of both groups.
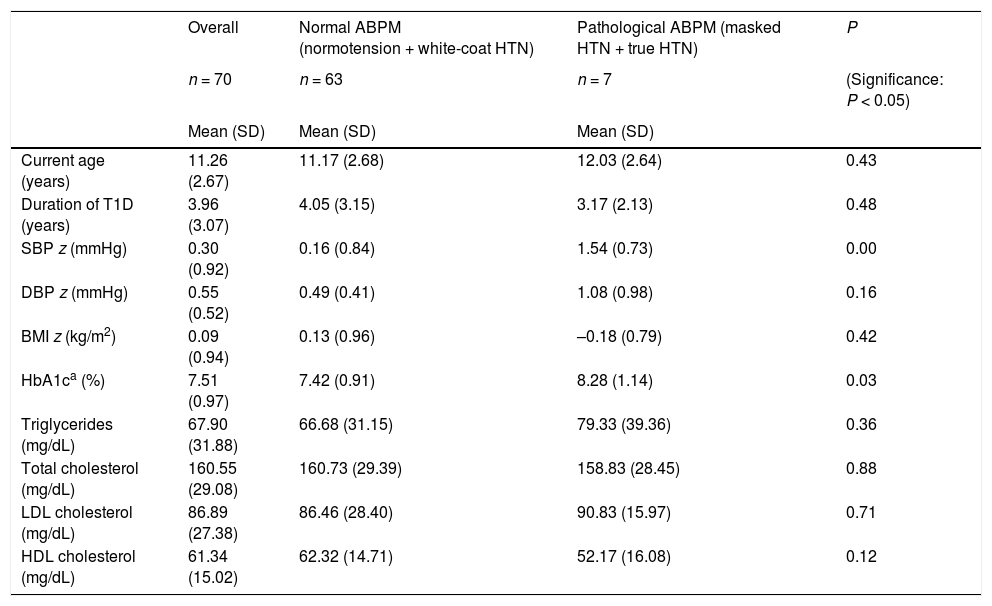
Mean age, time from onset, SBP z-score, DBP z-score, BMI z-score, HbA1c and lipid profile values in patients with normal and high ambulatory blood pressure values.
| Overall | Normal ABPM (normotension + white-coat HTN) | Pathological ABPM (masked HTN + true HTN) | P | |
|---|---|---|---|---|
| n = 70 | n = 63 | n = 7 | (Significance: P < 0.05) | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Current age (years) | 11.26 (2.67) | 11.17 (2.68) | 12.03 (2.64) | 0.43 |
| Duration of T1D (years) | 3.96 (3.07) | 4.05 (3.15) | 3.17 (2.13) | 0.48 |
| SBP z (mmHg) | 0.30 (0.92) | 0.16 (0.84) | 1.54 (0.73) | 0.00 |
| DBP z (mmHg) | 0.55 (0.52) | 0.49 (0.41) | 1.08 (0.98) | 0.16 |
| BMI z (kg/m2) | 0.09 (0.94) | 0.13 (0.96) | –0.18 (0.79) | 0.42 |
| HbA1ca (%) | 7.51 (0.97) | 7.42 (0.91) | 8.28 (1.14) | 0.03 |
| Triglycerides (mg/dL) | 67.90 (31.88) | 66.68 (31.15) | 79.33 (39.36) | 0.36 |
| Total cholesterol (mg/dL) | 160.55 (29.08) | 160.73 (29.39) | 158.83 (28.45) | 0.88 |
| LDL cholesterol (mg/dL) | 86.89 (27.38) | 86.46 (28.40) | 90.83 (15.97) | 0.71 |
| HDL cholesterol (mg/dL) | 61.34 (15.02) | 62.32 (14.71) | 52.17 (16.08) | 0.12 |
ABPM, ambulatory blood pressure monitoring; BMI, body mass index; HbA1c, glycated haemoglobin; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation; T1D, type 1 diabetes.
Ambulatory blood pressure monitoring is the gold standard for diagnosis of HTN in children with T1D.5,6 In the sample under study, ABPM proved very useful, for while the SBP z-score was significantly associated with high ambulatory BP, without ABPM cases of white-coat HTN would have not been identified and, more importantly, the diagnosis of 4 cases of masked HTN would have been missed. The accurate identification of HTN by ABPM has significant repercussions for treatment, as the current recommendation is that these patients initiate treatment with a combination of pharmacological and dietary measures.3 On the other hand, ABPM allows identification of abnormal BP patterns at night, which has been found to be associated with future development of albuminuria.7 When it came to the differences between the 2 groups, although excess weight is a risk factor for HTN, we did not observe differences in BMI between the groups. Patients with ambulatory HTN exhibited poorer glycaemic control, an association that has been described in the past.8
Although the sample size was small, our findings support the routine use of ABPM, especially in patients with poor glycaemic control. In all likelihood, once ABPM becomes more widely available, it will be used routinely following diagnosis of T1D.
Please cite this article as: Blázquez Gómez CJ, Alonso Rubio P, Megido Armada A, Huidobro Fernández B, Riaño Galán I. ¿Aporta la realización de monitorización ambulatoria de la presión arterial frente a la toma aislada en los pacientes pediátricos con diabetes tipo 1? An Pediatr (Barc). 2021;95:269–271.