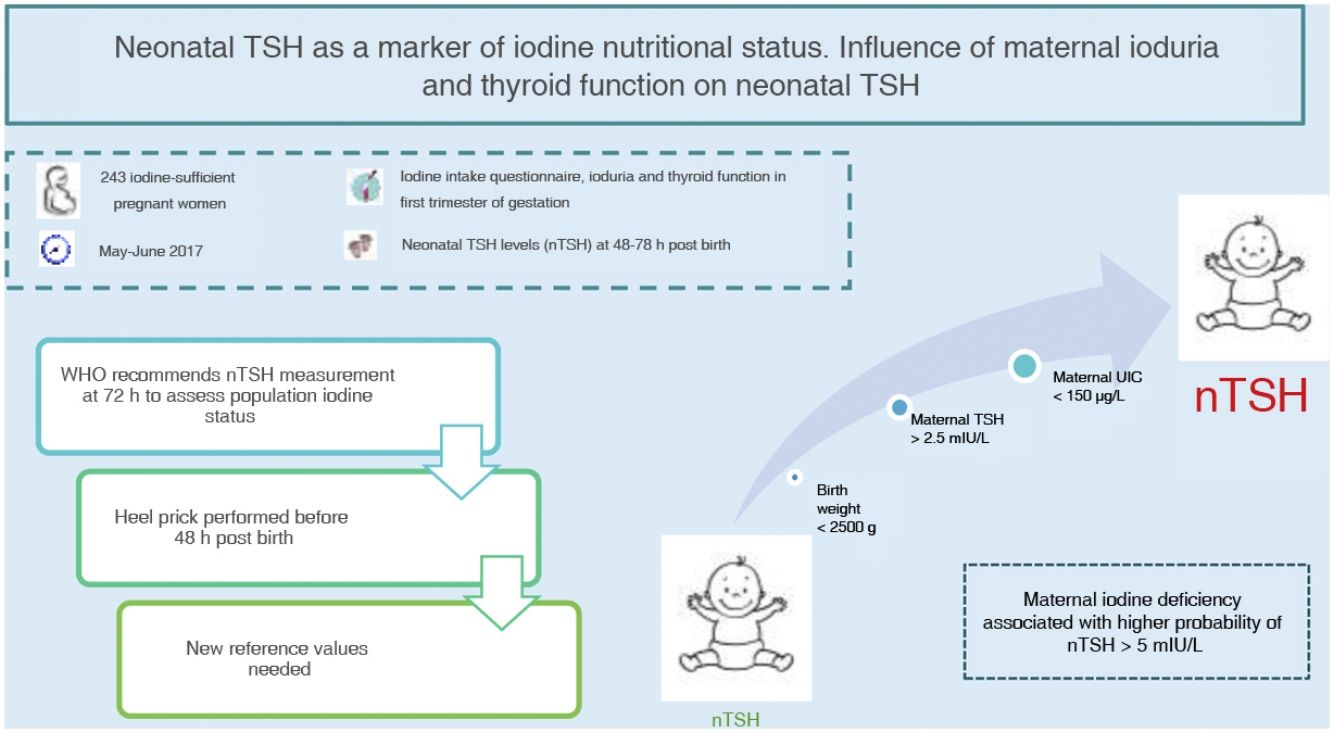
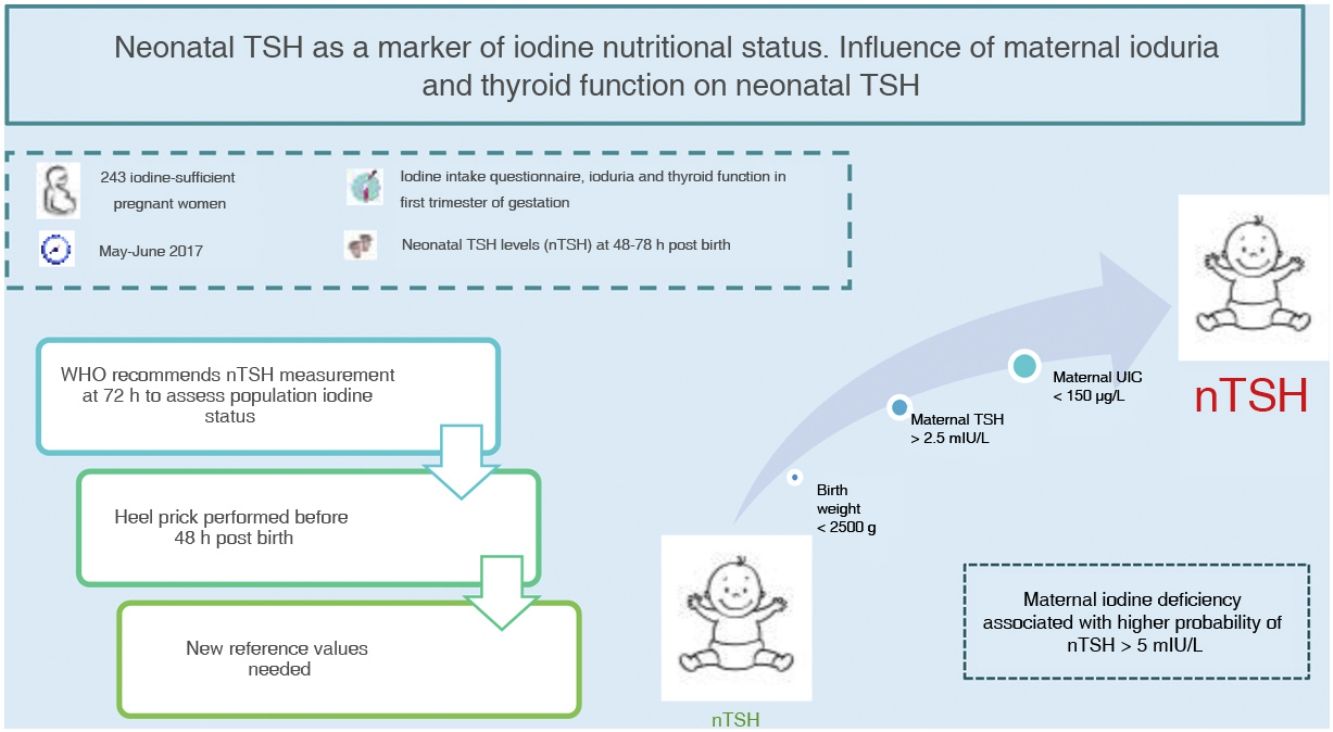
Neonatal thyroid stimulating hormone (nTSH) is a marker of iodine nutrition status in the population. The WHO considers a prevalence of less than 3% of nTSH levels greater than 5 mIU/L in samples obtained within 72h from birth indicative of iodine sufficiency. The aim of this study was to determine the prevalence of nTSH levels greater than 5 mIU/L in an iodine-sufficient population and its association with maternal, neonatal and obstetric factors.
Materials and methodsA total of 243 pregnant women were recruited between May and June 2017 in our health area. A questionnaire of iodine intake was administered, in addition to determination of ioduria, thyroid function and autoimmunity in the first trimester of gestation. We analysed nTSH levels in samples collected between 48 and 72h post birth and other obstetric and neonatal factors.
ResultsThe mean nTSH level (standard deviation) was 2.43 (1.68 mIU/L), with 7.8% of neonates having levels greater than 5 mIU/L. The highest nTSH levels corresponded to neonates of mothers with insufficient ioduria (P = 0.021) or TSH levels greater than 2.5 mIU/L, in both the case of negative (P = 0.049) and positive (P = 0.006) thyroid autoimmunity results. Maternal ioduria less than 150 μg/L was a risk factor for nTSH levels greater than 5 mIU/L (3.70 [1.06−14.60]; P = 0.046), while a neonatal weight of 2500 g or greater was a protective factor (0.14 [0.02−1.00]; P = 0.038).
ConclusionsThe prevalence of nTSH levels greater than 5 mIU/L in our health area was high based on the WHO recommendations. Maternal iodine deficiency was associated with a higher risk of nTSH levels greater than 5 mIU/L. Given that nTSH is currently measured before 72h post birth, we need new cut-off points to keep on using nTSH as a marker of iodine nutritional status.
La TSH neonatal (TSHn) es un marcador de nutrición de yodo en la población. La OMS relaciona una prevalencia <3% de TSHn >5 mUI/L, obtenidas a partir de las 72h del nacimiento, con un adecuado estado nutricional de yodo. El objetivo de este estudio es conocer la prevalencia de TSHn >5 mUI/L en una población yodosuficiente y su relación con factores maternos, neonatales y obstétricos.
Materiales y métodosSe reclutaron 243 gestantes entre mayo y junio de 2017 en nuestra área sanitaria. Se realizó un cuestionario sobre consumo de yodo y determinación de yoduria, función y autoinmunidad tiroideas en el primer trimestre de gestación. Se analizó la TSHn entre 48−72h del nacimiento, así como otros factores obstétricos y neonatales.
ResultadosLa TSHn media fue 2,43 ± 1,68 mUI/L, con 7,8% de neonatos con TSHn >5 mUI/L. La TSHn más elevada pertenecía a los neonatos de madres con yodurias insuficientes (p = 0.021) o con TSH > 2.5 mUI/L, tanto en autoinmunidad tiroidea negativa (p = 0,049) como positiva (p = 0,006). La yoduria materna <150 μg/L fue un factor de riesgo de TSHn >5 mUI/L (3,70 [1,06-14,60], p = 0,046), mientras el peso neonatal ≥2500 gr fue un factor protector (0,14 [0,02-1,00], p = 0,038).
ConclusionesLa prevalencia de TSHn >5 mUI/L en nuestra área sanitaria fue elevada, según las recomendaciones de la OMS. Se asoció el déficit de yodo materno con mayor riesgo de TSHn >5 mUI/L. Dado que en la actualidad la determinación de la TSHn se realiza antes de las 72h del nacimiento, precisamos de nuevos puntos de corte para continuar empleando la TSHn como marcador de nutrición de yodo.
Iodine is an essential trace element for the synthesis of thyroid hormones. In adults, iodine deficiency is associated with thyroid dysfunction and goitre, and in pregnant women, with increases in the frequency of miscarriage, perinatal mortality and congenital anomalies in the offspring1, which in turn is associated with abnormal growth and neurodevelopment2. Iodine deficiency continues to be the leading cause of preventable neurologic impairment3. The World Health Organization (WHO) recommends universal salt iodization and the use of iodine supplements in at-risk groups, in addition to periodic performance of surveys to monitor iodine status at the population level4.
Measurement of the urinary iodine concentration (UIC) in school-age children is the most widely used method to assess population iodine status5. However, other markers are also available, such as neonatal thyroid stimulating hormone (TSH) levels4,6,7, the prevalence of goitre in school-age children6 or thyroglobulin serum levels8.
Neonatal TSH levels are used to screen congenital hypothyroidism in newborns, but is also a good marker of iodine intake and status, as the low concentration of iodine in the neonatal thyroid gland requires higher rates of iodine turnover, so that TSH levels increase if the iodine supply is low3,4. Based on the recommendations of the WHO, a prevalence of less than 3% of neonatal TSH levels greater than 5 mIU/L is indicative of iodine sufficiency in a population4. However, numerous obstetric and neonatal factors can influence neonatal TSH levels besides maternal iodine status9,10.
The main aim of our study was to determine the prevalence of neonatal TSH levels greater than 5 mIU/L in our catchment area. A secondary objective was to assess the impact of maternal iodine intake, UIC and thyroid on neonatal TSH levels. Lastly, we analysed other obstetric and neonatal factors that could be associated with neonatal TSH levels.
Material and methodsStudy populationWe conducted a longitudinal, observational, descriptive and analytic study in pregnant women and neonates in the hospital’s catchment area, with a population of 330,560 inhabitants. Overall, iodine status in this region is adequate, both in the general population11 and in pregnant women12.
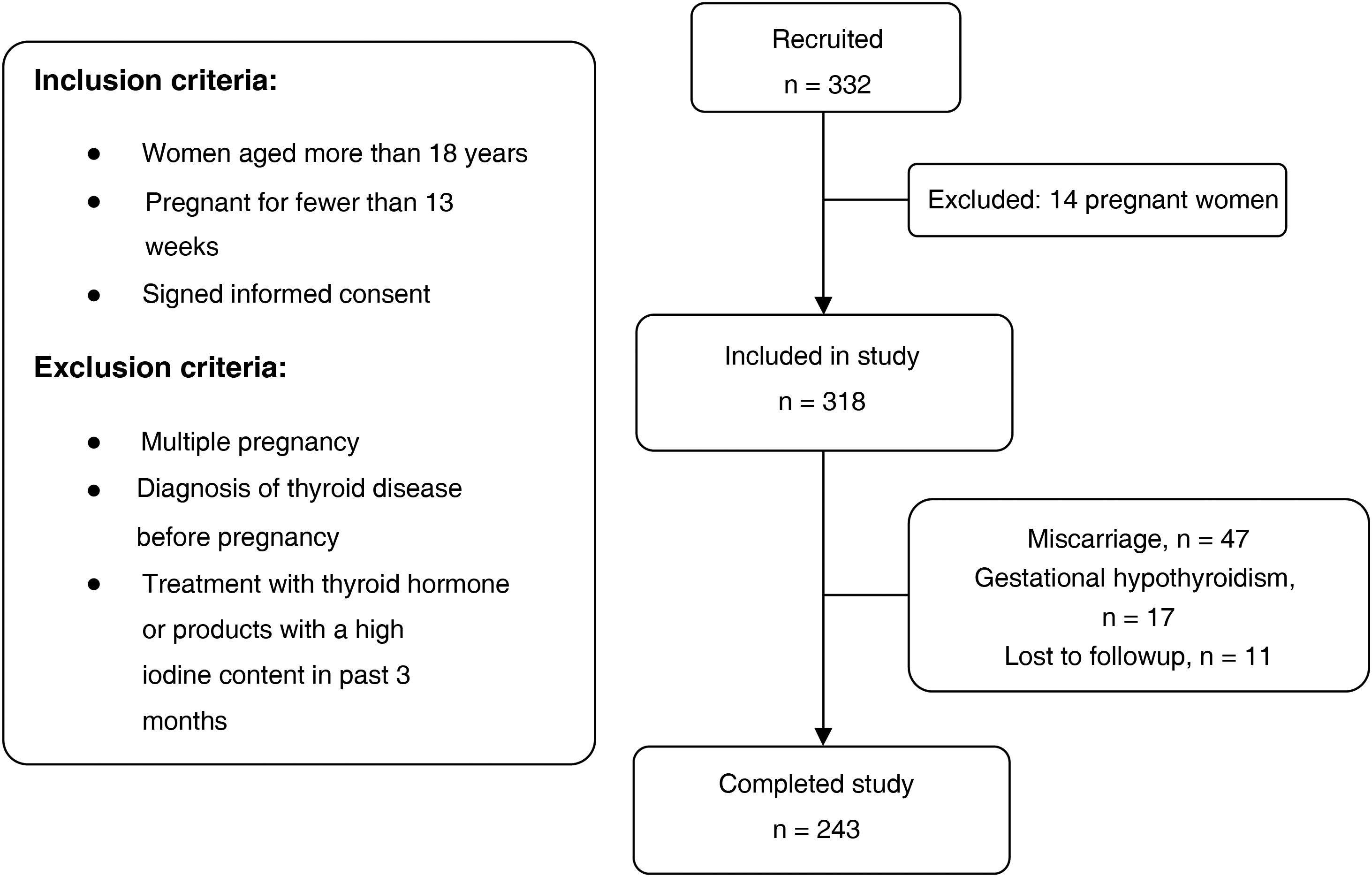
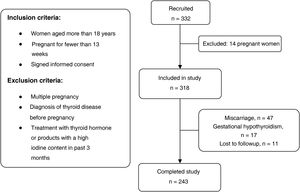
Participants were selected out of the total of pregnant women who attended the initial visit with the midwife between May and June 2017. The applied inclusion and exclusion criteria can be seen in the flow chart of the study (Fig. 1).
The study was approved by the Regional Research Ethics Committee. All participants signed the informed consent form.
Study variablesIn the first visit with the midwife, a questionnaire was administered to assess iodine intake, collecting information on the regular consumption of iodized salt (yes/no) and dairy products (daily servings of milk, yoghurt and cheese), and use of iodine supplementation (yes/no). The results of the iodine intake survey were published by González et al.12.
Urinary iodine concentration, thyroid function and antithyroid-antibody tests were performed in the first trimester of gestation, the results of which were published by González et al.12, and we also collected neonatal TSH levels.
The maternal UIC was measured in a random urine sample by inductively coupled plasma mass spectrometry (ICP-MS) with an Agilent 7700× spectrometer (Agilent Technologies, Santa Clara, CA, USA.). This method exhibited an adequate linearity between 10 and 450 μg/L (R2 > 0.99), with an intralaboratory imprecision of 2.9% or less and a total analytical error of 7.3% or less.
In the same visit, the mother underwent collection of a blood sample for measurement of TSH levels and anti-thyroid antibody testing (thyroid peroxidase antibodies [TPOAb] and thyroglobulin antibodies [TgAb]). The measurements were made by electrochemiluminescence immunoassay (Roche Diagnostics, Basel, Switzerland). The coefficient of variation (CV) for TSH values ranged from 0.8% to 2.9%. In our catchment area, the normal range for TSH levels was 0.20–4.50 mIU/L. The normal range for autoantibodies was less than 34 UI/mL for TPOAb and less than 18 UI/mL for TgAb.
Newborns underwent heel lancing, preferably between 48 and 72h post birth, for collection of capillary blood collected in a Whatman® 903 paper card (dried blood spot) in the framework of the congenital hypothyroidism screening programme. Neonatal TSH was measured by dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA) in the clinical biochemistry laboratory of our hospital. We considered neonatal TSH levels of less than 10 mIU/L normal. If the value was between 10 and 20 mIU/L, a second measurement was made, and in the case of a TSH level greater than 20 mIU/L, the patient was referred to the neonatal unit.
Lastly, we collected data on the birth weight and gestational age of the newborn, mode of delivery (uncomplicated [not requiring intervention by physician/midwife], operative vaginal delivery or caesarean section), reason for operative vaginal or caesarean delivery, presence of nonreassuring foetal status (NRFS) and 1-min Apgar score (Apgar-1). The presence of NRFS was determined by the obstetrician if there were any signs or symptoms suggestive of hypoxic metabolic acidosis, including severe clinical conditions and abnormalities in the different diagnostic methods used to assess foetal wellbeing, such as cardiotocography, pulse oximetry, pH measurement or Doppler ultrasound.
Statistical analysisWe conducted a descriptive analysis, calculating absolute and relative frequency distributions for qualitative variables and measures of central tendency and dispersion for quantitative variables. We assessed differences in numerical variables by means of the Student t-test or Wilcoxon test for independent samples based on whether the homogeneity of variance assumption was met. To compare three or more variables, we used analysis of variance (ANOVA) or the Kruskal-Wallis test, based on whether the normality and homoscedasticity assumptions were met. We assessed the association between two variables by means of the χ2 test or Fisher exact test based on the expected counts. We used the McNemar test in the case of paired proportions. We fitted 2 multivariate binary logistic regression models to predict neonatal levels of TSH greater than 5 mIU/L, calculating odds ratios (OR), 95% confidence intervals (CIs) and P values for the Wald test.
We entered the data in an ACCESS-SQL 2010 database. The statistical analysis was performed with the software R (R Development Core Team), version 36.0. We defined statistical significance as a P value of 005.
ResultsWe selected 332 pregnant women, of who 14 were not eligible because they met 1 or more of the exclusion criteria. Other patients did not complete the study due to miscarriage ( = n 47), diagnosis of gestational hypothyroidism (n = 17) or being lost to follow-up (n = 11). Ultimately, we collected data for 243 mother-child dyads.
Out of all neonates, 124 were male. The mean gestational age was 39.8 weeks (standard deviation [SD], 1.6), and 13 infants were born preterm (<37 weeks of gestation). The mean birth weight was 3260 ± 517.5 g, and 16 neonates had birth weights of less than 2500 g.
Neonatal TSH levelsNeonatal TSH levels were measured in samples collected between 48 and 72h post birth in 90.4% of newborns (95th percentile [P95], 72h).
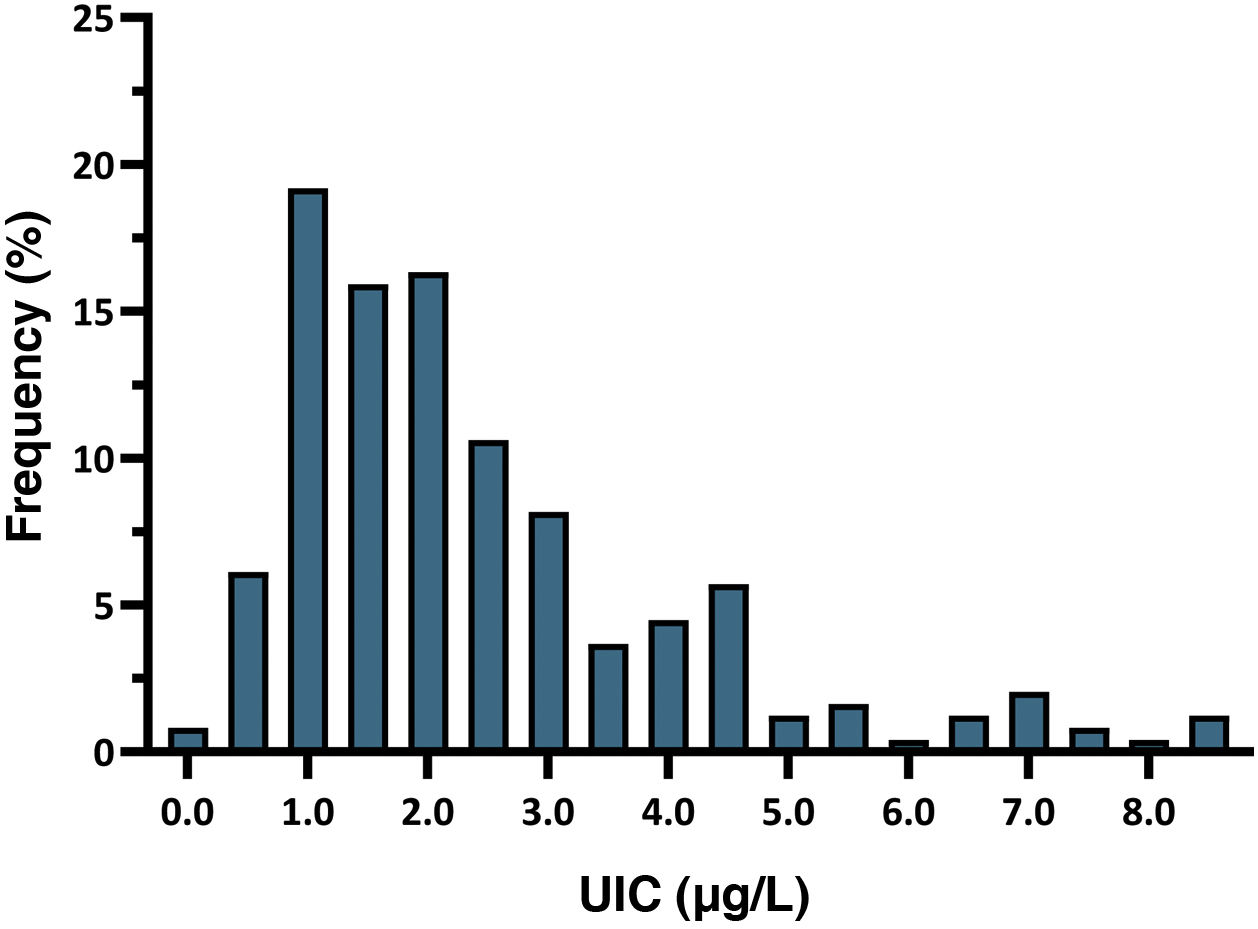
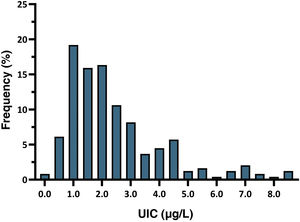
The mean neonatal TSH level was 2.43 mIU/L (SD, 1.68). The 5th to 95th percentile range was 0.60–6.60 mIU/L, while the first to 99th percentile range was 0.25–8.60 mIU/L. All newborns had TSH levels in the normal range, and 7.8% had levels greater than 5 mIU/L. Fig. 2 presents the distribution of neonatal TSH levels.
We analysed neonatal TSH values based on the timing of sample collection. The mean TSH value in samples collected within 48h of birth was 3.36 mIU/L (SD, 1.83 mIU/L), significantly higher compared to samples collected between 48 and 72h (mean, 2.44 mIU/L; SD, 1.69) or 72h post birth (1.79 mIU/L; SD, 1.80 mIU/L) (P = 0.020).
Maternal factors: iodine intake, ioduria and thyroid functionWe analysed the association between iodine intake (iodized salt, dairy products and iodine supplements) and neonatal TSH levels, and found no statistically significant differences.
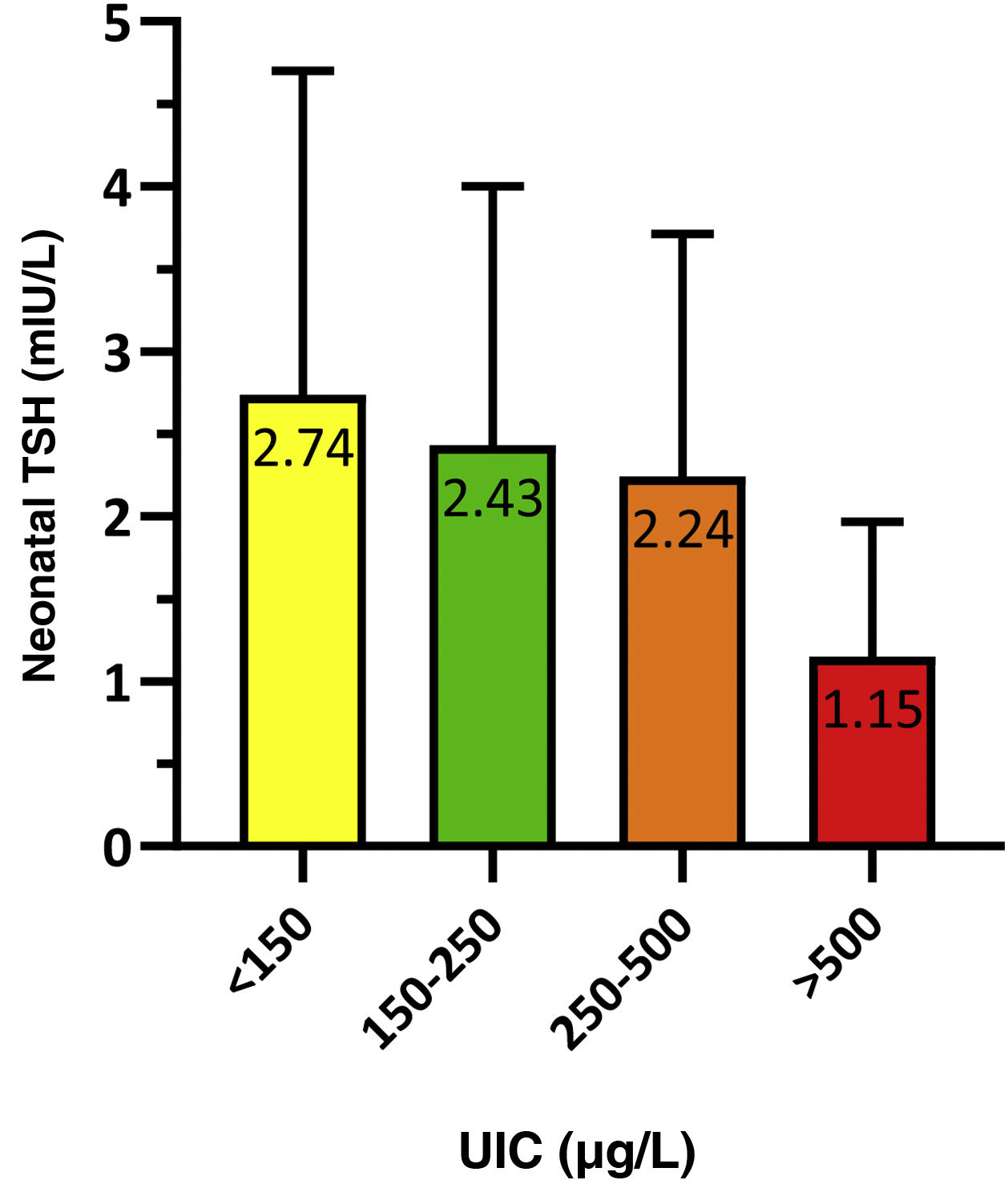
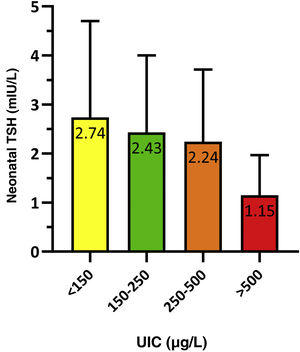
To analyse the association between neonatal TSH and maternal UIC in the first trimester of gestation, we grouped newborns based on whether their mothers had an insufficient (<150 μg/L), adequate (150−250 μg/L), greater than adequate (250−500 μg/L) or excessive (>500 μg/L) concentration of iodine in urine. The results were statistically significant (P = 0.021) and are presented in Fig. 3.
We calculated the proportion of infants with neonatal TSH levels greater than 5 mIU/L based on the maternal UIC, and found statistically significant differences (p = 0.014). The proportion of infants with TSH values greater than 5 mIU/L was 11.9% in the insufficient maternal UIC group compared to 8.3% in the adequate maternal UIC group. All infants born to mothers with an UIC greater than 250 μg/L had neonatal TSH values of less than 5 mIU/L.
The analysis of the association of neonatal TSH values and maternal thyroid function was limited to the cases for which antithyroid-antibody test results were available (n = 128). Table 1 summarises these results. We found that the mean neonatal TSH level was significantly lower in infants born to mothers with TSH values of less than 2.50 mIU/L, independently of thyroid autoantibody status, although the differences were more marked in the subset with mothers who tested positive for thyroid autoantibodies. All infants born to mothers with TSH levels of less than 2.50 and negative thyroid autoantibody test results had neonatal TSH levels of 5 mIU/L or less.
Association between maternal and neonatal TSH levels expressed as mean and standard deviation.
| Maternal TSH (mIU/L) | n | Neonatal TSH (mIU/L) | P | |
|---|---|---|---|---|
| Negative thyroid autoantibodies | <2.50 | 56 | 2.05 ± 1.33 | 0.049 |
| ≥2.50 | 36 | 2.78 ± 1.92 | ||
| Positive thyroid autoantibodies | <2.50 | 20 | 1.47 ± 1.44 | 0.006 |
| ≥2.50 | 16 | 2.73 ± 1.47 |
Fifty-six percent of infants were born by uncomplicated vaginal delivery, 28.4% by caesarean delivery and 15.6% by operative vaginal delivery. The mean neonatal TSH level was 2.04 mIU/L (SD, 1.52) in the uncomplicated delivery group, 3.04 mIU/L (SD, 1.79) in the caesarean delivery group and 2.88 mIU/L (SD, 1.72) in the operative vaginal delivery group (P < 0.001).
In the uncomplicated delivery group, 4.4% of infants had neonatal TSH levels greater than 5 mIU/L, compared to 7.9% in the caesarean delivery group and 14.5% in the operative vaginal delivery group (P = 0.039).
Birth weight and gestational ageWe compared neonatal TSH levels in infants with birthweights of less than 2500 g and infants with birthweights of 2500 g and greater, and found no significant differences (mean ± SD, 2.73 ± 2.71 mIU/L vs 2.41 ± 1.59 mIU/L; P = 0.345).
We also compared neonatal TSH levels in infants born before and after 37 weeks’ gestation, and also found no significant differences (mean ± SD, 3.34 ± 3.17 mIU/L vs 2.40 ± 1.60 mIU/L; P = 0.224).
Probability of nonreassuring foetal statusNonreassuring foetal status was documented in 24.7% of newborns. The proportion of NRFS was 31.2% in infants with birthweights of less than 2500 g compared to 19.8% in those with birthweights of 2500 g or greater. Also, NRFS occurred in 23.1% of infants born before 37 weeks compared to 24.8% of those born at 37 weeks or after. Nonreassuring foetal status was the most frequent reason for caesarean delivery (41.5% of total cases) and operative vaginal delivery (64.2% of total cases).
We compared neonatal TSH levels in infants with a history of NRFS to neonatal TSH levels in infants born by caesarean section or instrumental delivery without NRFS and infants born by uncomplicated vaginal delivery. The mean neonatal TSH values in these groups were 3.22 mIU/L (SD, 1.84), 2.67 mIU/L (SD, 1.75) and 2.02 mIU/L (SD, 1.52), respectively (P < 0.001). Fifteen percent of infants born by caesarean section or operative vaginal delivery due to NRFS had neonatal TSH levels greater than 5 mIU/L compared to 10.4% of infants born by caesarean section or operative vaginal delivery without NRFS and 4.3% of infants with an uncomplicated vaginal birth (P = 0.026).
1-min APGARThe 1-min Apgar score was less than 9 points in 20.2% of the infants. The neonatal TSH levels in these patients were greater compared to those with 1-min Apgar scores of 9 or more points (mean ± SD, 2.80 ± 1.84 mIU/L vs 2.34 ± 1.63 mIU/L), although the difference was not statistically significant (P = .087). Of the infants with 1-min Apgar scores of less than 9 points, 16.3% had neonatal TSH levels greater than 5 mIU/L compared to 5.7% of infants with 1-min Apgar scores of 9 points or greater (P = .031). The mean 1-min Apgar score in newborns with neonatal TSH levels of 5 mIU/L or lower was greater compared to newborns with TSH levels greater than 5 mIU/L (mean ± SD, 8.80 ± 0.91 points vs 7.84 ± 1.74 points; P = 0.002).
Multivariate analysisWe conducted a univariate analysis and fitted 2 multivariate binary logistic regression models (Table 2). The dependent variable in both models was a neonatal TSH level greater than 5 mIU/L. In Model 1, the included variables were maternal UIC, thyroid function and iodine intake. This initial analysis showed that a maternal UIC greater than 150 μg/L was a risk factor for a neonatal TSH level greater than 5 mIU/L (OR, 3.27; 95% CI, 1.03−11.55; P = 0.049). In Model 2, in addition to these variables, we included the mode of delivery, birth weight, gestational age, presence of NRFS and 1-min Apgar score. In this model, a maternal UIC greater than 150 μg/L remained a risk factor for a neonatal TSH level greater than 5 mIU/L (OR, 3.70; 95% CI, 1.06−14.60; P = 0.046), while a birthweight of 2500 g or greater was a protective factor (OR, 0.14; 95% CI, 0.02−1.00; P = 0.038). A 1-min Apgar score of less than 9 points behaved as a protective factor against neonatal TSH levels greater than 5 mIU/L, with a P value that neared the threshold of significance.
Univariate and multivariate binary logistic regression analysis with neonatal TSH greater than 5 mIU/L as the dependent variable.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Model 1 | ||||||
| Iodized salt | ||||||
| No | – | – | – | – | – | – |
| Yes | 0.83 | 0.29−2.44 | 0.723 | 1.30 | 0.42−4.25 | 0.655 |
| Dairy products | ||||||
| <2 servings | – | – | – | – | – | – |
| ≥2 servings | 0.86 | 0.30−2.38 | 0.767 | 0.80 | 0.22−2.62 | 0.718 |
| Iodine supplement | ||||||
| No | – | – | – | – | – | – |
| Yes | 0.92 | 0.24−6.05 | 0.913 | 0.76 | 0.15−5.66 | 0.758 |
| UIC (μg/L) | ||||||
| ≥150 | – | – | – | – | – | – |
| <150 | 3.06 | 1.18−8.53 | 0.024 | 3.27 | 1.03−11.55 | 0.049 |
| TSH (mIU/L) | ||||||
| <2.50 | – | – | – | – | – | – |
| ≥2.50 | 0.90 | 0.31−2.37 | 0.835 | 1.00 | 0.26−3.29 | 0.995 |
| Model 2 | ||||||
| Iodized salt | ||||||
| No | – | – | – | – | – | – |
| Yes | 0.83 | 0.29−2.44 | .723 | 1.48 | 0.42−5.58 | 0.548 |
| Dairy products | ||||||
| <2 servings | – | – | – | – | – | – |
| ≥2 servings | 0.86 | 0.30−2.38 | 0.767 | 0.76 | 0.19−2.82 | 0.690 |
| Iodine supplement | ||||||
| No | – | – | – | – | – | – |
| Yes | 0.92 | 0.24−6.05 | 0.913 | 1.05 | 0.16−10.09 | 0.961 |
| UIC (μg/L) | ||||||
| ≥150 | – | – | – | – | – | – |
| <150 | 3.06 | 1.18−8.53 | 0.024 | 3.70 | 1.06−14.60 | 0.046 |
| TSH (mIU/L) | ||||||
| <2.50 | – | – | – | – | – | – |
| ≥2.50 | 0.90 | 0.31−2.37 | 0.835 | 0.77 | 0.17−2.88 | 0.715 |
| Delivery | ||||||
| Uncomplicated | – | – | – | – | – | – |
| Caesarean | 1.86 | 0.38−7.42 | 0.398 | 1.87 | 0.21−12.21 | 0.527 |
| Operative vaginal | 3.67 | 1.30−11.24 | 0.016 | 3.76 | 0.78−18.43 | 0.093 |
| Peso neonatal (g) | ||||||
| <2500 | – | – | – | – | – | – |
| ≥2500 | 0.33 | 0.09−1.54 | 0.107 | 0.14 | 0.02−1.0 | 0.038 |
| Gestational age (weeks) | ||||||
| <37 | – | – | – | – | – | – |
| ≥37 | 0.44 | 0.11−3.00 | 0.312 | 2.14 | 0.19−60.45 | 0.584 |
| NRFS | ||||||
| No | – | – | – | – | – | – |
| Yes | 2.03 | 0.72−5.33 | 0.158 | 0.47 | 0.09−2.16 | 0.340 |
| 1-min Apgar (points) | ||||||
| <9 | – | – | – | – | – | – |
| ≥9 | 0.31 | 0.12−0.84 | 0.018 | 0.28 | 0.07−1.04 | 0.054 |
CI, confidence interval; UIC, urinary iodine concentration; NRFS, nonreassuring foetal status; OR, odds ratio; TSH, thyroid stimulating hormone.
In our study, the mean neonatal TSH level was 2.43 mIU/L, and none of the newborns had TSH levels greater than 10 mIU/L. The proportion of newborns with TSH values greater than 5 mIU/L was 7.8%. The WHO has proposed that a frequency of 3% or greater of neonatal TSH levels over 5 mIU/L is indicative of iodine insufficiency in the population4, a criterion that has since been validated by Zimmermann et al.7. However, pregnant women in our study exhibited an adequate iodine status12 and the general population in our area is also iodine sufficient11.
The main factor that may explain a high prevalence of neonatal TSH levels greater than 5 mIU/L in an iodine-sufficient population is the time elapsed from birth to collection of the blood sample. The foetal thyroid starts to synthesise hormones from 18 to 20 weeks of gestation2. From that point, TSH levels increase progressively, peaking 30−60 min post birth at values of up to 60–100 mIU/L, followed by a marked drop in the next 48 h and eventual stabilization by 3–5 days post birth13,14. The WHO recommends measurement of neonatal TSH levels in dried blood spots with collection of sample from 72h post birth4. However, in our study, as is the case of many other recent ones, samples were collected in the framework of newborn screening for congenital hypothyroidism between 48 and 72h post birth. Our findings corroborate that neonatal TSH levels continue to decline past 72h post birth.
Measuring neonatal TSH levels is the most sensitive method to identify congenital hypothyroidism15, but these values may be affected by several factors9,10. One is the maternal iodine status. The foetal thyroid is very sensitive to iodine deficiency, but also to its excess. When iodine levels are excessive, the foetal thyroid is not sufficiently mature to escape the inhibitory Wolff-Chaikoff effect until 36 weeks’gestation16,17. This is reflected by multiple studies in which excessive iodine intake in the mother was found to be associated with congenital hypothyroidism17,18 or the use of iodine-containing antiseptics during childbirth was found to induce transient hypothyroidism in neonates19.
In our study, we did not find a significant association between neonatal TSH levels and maternal iodine intake, but we found an association with the maternal UIC in the first trimester of the pregnancy. The offspring of mothers with sufficient UICs did not have neonatal TSH levels greater than 5 mIU/L and, overall, neonatal TSH levels were higher the lower the maternal UIC. Both multivariate models showed that maternal iodine deficiency is a risk factor for neonatal TSH levels greater than 5 mIU/L, corroborating the association between maternal iodine status and neonatal TSH levels. The results of previous studies have been heterogeneous, with some confirming20,21 and others finding no evidence22,23 of this association.
Another factor that may have an impact on neonatal TSH levels is maternal thyroid function22,24. In our study, we found that infants born to mothers with TSH levels of less than 2.50 mIU/L had significantly lower neonatal TSH levels compared to infants born to mothers with TSH levels above that threshold, with more marked differences in the case of mothers who were positive for thyroid autoantibodies. In addition, all infants born to mothers with TSH levels under TSH <2.5 mIU/L and negative for thyroid autoantibodies had neonatal TSH levels of 5 mIU/L or less.
In addition to being influenced by the iodine status or thyroid function of the mother, neonatal TSH levels may be affected by multiple maternal, neonatal or obstetric factors resulting in foetal stress14,25. The mode of delivery, preterm birth or low birth weight for gestational age are some of these factors.
Caesarean delivery is associated with lower neonatal TSH levels compared to operative or uncomplicated vaginal delivery14,26. These differences can be explained by the increased catecholamine levels associated with vaginal delivery9,14. Still, there have also been studies that have not found any differences based on the mode of delivery22,27 and others in which the highest neonatal TSH levels were found in infants delivered by caesarean section28,29. In our study, infants delivered by caesarean section had the highest neonatal TSH levels, although the multivariate analysis did not find an association between mode of delivery and neonatal TSH levels.
Congenital hypothyroidism is more frequent in preterm or low birth weight newborns due to the immaturity of the hypothalamic-pituitary axis30. Preterm infants may experience transient hypothyroxinaemia in the first weeks of life, with a delayed postnatal TSH peak that usually takes place 2–6 weeks post birth2,31. Low birth weight is also an independent factor that may influence neonatal TSH levels28,32. In our study, a birth weight greater than 2500 g was associated with a lower probability of neonatal TSH levels greater than 5 mIU/L, although we did not find an association with gestational age.
Lastly, we analysed 2 factors that are direct indicators of foetal stress, such as nonreassuring foetal status and the 1-min Apgar score. Neither of these factors was significantly associated with TSH levels in our study, but Korevaar et al.24 found that factors related to foetal stress, such as respiratory distress, were associated with transient abnormalities in neonatal thyroid function. Along the same lines, other studies have found an association between foetal distress33 or low Apgar scores34 and abnormal thyroid function in the neonatal period.
The main limitation of our study was the sample size which, while large enough to assess the primary outcomes, was too small to yield statistically significant results in some of the analyses. Another limitation was the retrospective data collection, as there could have been confounding factors at play that were not taken into account and interfered with the results.
In conclusion, the prevalence of neonatal levels of TSH greater than 5 mIU/L in our catchment area was high based on the recommendations of the WHO, in spite of being a population with an adequate iodine status. Neonatal TSH levels are a good marker of iodine nutritional status, as demonstrated by their correlation with maternal urinary iodine concentrations in the first trimester of gestation, and maternal iodine deficiency was associated with an increased risk of neonatal TSH levels greater than 5 mIU/L. However, since neonatal TSH levels are currently measured within 72h of birth in the context of screening for congenital hypothyroidism, new cut-off points need to be established to continue using this parameter as a marker of iodine status, which requires performance of additional studies.
Conflicts of interestThe authors have no conflicts of interest to declare.