In recent years there has been an increase in the frequency of diagnosis of neonatal thrombosis associated with the increased use of imaging tests1 and the increased survival of patients with complex conditions. The incidence is of 5 per 100,000 live births and 5 per 1000 patients admitted to an intensive care unit.2 Central catheterization is a risk factor found in 90% of episodes.3 At present, the treatment of thrombosis in newborns is based on adult guidelines4 and therefore a subject of controversy, with considerable heterogeneity in its management in clinical practice.
We describe the cases of 29 patients with thrombosis managed between 2008 and 2017 in a tertiary level neonatal intensive care unit (excluding postsurgical cardiac patients or patients treated with extracorporeal membrane oxygenation, which was delivered in a different unit of the hospital).
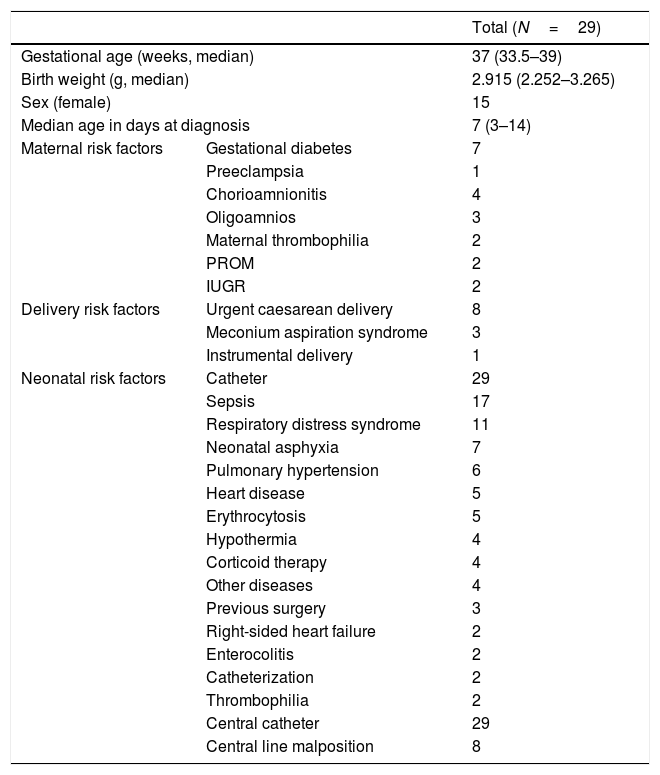
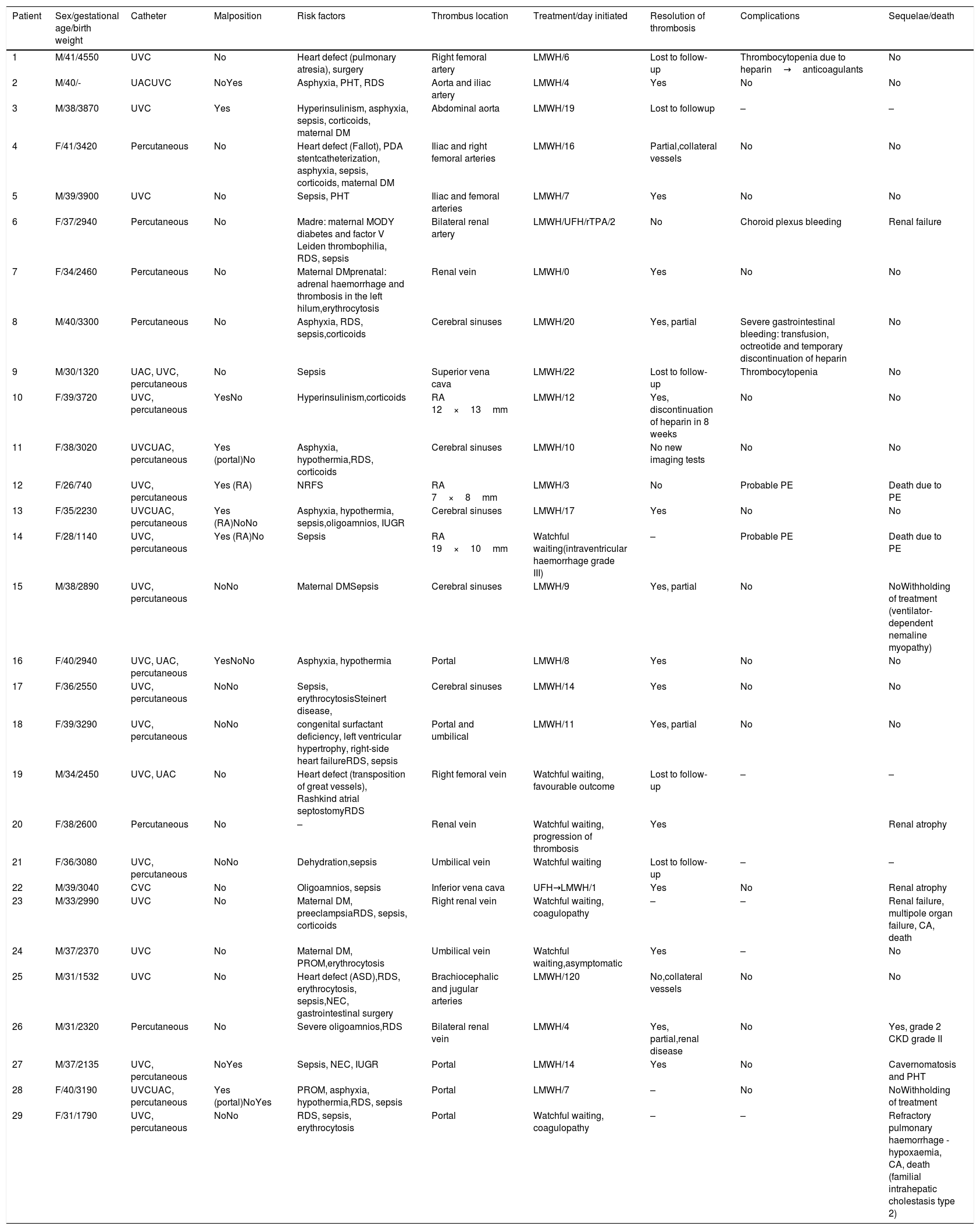
Table 1 presents the epidemiological characteristics and risk factors of the patients included in the study. The clinical manifestations that preceded diagnosis were heterogeneous, and 38.4% of the patients were asymptomatic. The diagnosis was based on the findings of Doppler ultrasound in 26 cases, and on the findings of magnetic resonance imaging (MRI) or computed tomography (CT) scans in the remaining 3. Table 2 presents the location of the thrombus, risk factors, treatment and outcome for each case.
Patient characteristics and risk factors.
| Total (N=29) | ||
|---|---|---|
| Gestational age (weeks, median) | 37 (33.5–39) | |
| Birth weight (g, median) | 2.915 (2.252–3.265) | |
| Sex (female) | 15 | |
| Median age in days at diagnosis | 7 (3–14) | |
| Maternal risk factors | Gestational diabetes | 7 |
| Preeclampsia | 1 | |
| Chorioamnionitis | 4 | |
| Oligoamnios | 3 | |
| Maternal thrombophilia | 2 | |
| PROM | 2 | |
| IUGR | 2 | |
| Delivery risk factors | Urgent caesarean delivery | 8 |
| Meconium aspiration syndrome | 3 | |
| Instrumental delivery | 1 | |
| Neonatal risk factors | Catheter | 29 |
| Sepsis | 17 | |
| Respiratory distress syndrome | 11 | |
| Neonatal asphyxia | 7 | |
| Pulmonary hypertension | 6 | |
| Heart disease | 5 | |
| Erythrocytosis | 5 | |
| Hypothermia | 4 | |
| Corticoid therapy | 4 | |
| Other diseases | 4 | |
| Previous surgery | 3 | |
| Right-sided heart failure | 2 | |
| Enterocolitis | 2 | |
| Catheterization | 2 | |
| Thrombophilia | 2 | |
| Central catheter | 29 | |
| Central line malposition | 8 | |
IUGR, intrauterine growth restriction; PROM, premature rupture of membranes.
Risk factors, location, treatment and outcome.
| Patient | Sex/gestational age/birth weight | Catheter | Malposition | Risk factors | Thrombus location | Treatment/day initiated | Resolution of thrombosis | Complications | Sequelae/death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/41/4550 | UVC | No | Heart defect (pulmonary atresia), surgery | Right femoral artery | LMWH/6 | Lost to follow-up | Thrombocytopenia due to heparin→anticoagulants | No |
| 2 | M/40/- | UACUVC | NoYes | Asphyxia, PHT, RDS | Aorta and iliac artery | LMWH/4 | Yes | No | No |
| 3 | M/38/3870 | UVC | Yes | Hyperinsulinism, asphyxia, sepsis, corticoids, maternal DM | Abdominal aorta | LMWH/19 | Lost to followup | – | – |
| 4 | F/41/3420 | Percutaneous | No | Heart defect (Fallot), PDA stentcatheterization, asphyxia, sepsis, corticoids, maternal DM | Iliac and right femoral arteries | LMWH/16 | Partial,collateral vessels | No | No |
| 5 | M/39/3900 | UVC | No | Sepsis, PHT | Iliac and femoral arteries | LMWH/7 | Yes | No | No |
| 6 | F/37/2940 | Percutaneous | No | Madre: maternal MODY diabetes and factor V Leiden thrombophilia, RDS, sepsis | Bilateral renal artery | LMWH/UFH/rTPA/2 | No | Choroid plexus bleeding | Renal failure |
| 7 | F/34/2460 | Percutaneous | No | Maternal DMprenatal: adrenal haemorrhage and thrombosis in the left hilum,erythrocytosis | Renal vein | LMWH/0 | Yes | No | No |
| 8 | M/40/3300 | Percutaneous | No | Asphyxia, RDS, sepsis,corticoids | Cerebral sinuses | LMWH/20 | Yes, partial | Severe gastrointestinal bleeding: transfusion, octreotide and temporary discontinuation of heparin | No |
| 9 | M/30/1320 | UAC, UVC, percutaneous | No | Sepsis | Superior vena cava | LMWH/22 | Lost to follow-up | Thrombocytopenia | No |
| 10 | F/39/3720 | UVC, percutaneous | YesNo | Hyperinsulinism,corticoids | RA 12×13mm | LMWH/12 | Yes, discontinuation of heparin in 8 weeks | No | No |
| 11 | F/38/3020 | UVCUAC, percutaneous | Yes (portal)No | Asphyxia, hypothermia,RDS, corticoids | Cerebral sinuses | LMWH/10 | No new imaging tests | No | No |
| 12 | F/26/740 | UVC, percutaneous | Yes (RA) | NRFS | RA 7×8mm | LMWH/3 | No | Probable PE | Death due to PE |
| 13 | F/35/2230 | UVCUAC, percutaneous | Yes (RA)NoNo | Asphyxia, hypothermia, sepsis,oligoamnios, IUGR | Cerebral sinuses | LMWH/17 | Yes | No | No |
| 14 | F/28/1140 | UVC, percutaneous | Yes (RA)No | Sepsis | RA 19×10mm | Watchful waiting(intraventricular haemorrhage grade III) | – | Probable PE | Death due to PE |
| 15 | M/38/2890 | UVC, percutaneous | NoNo | Maternal DMSepsis | Cerebral sinuses | LMWH/9 | Yes, partial | No | NoWithholding of treatment (ventilator-dependent nemaline myopathy) |
| 16 | F/40/2940 | UVC, UAC, percutaneous | YesNoNo | Asphyxia, hypothermia | Portal | LMWH/8 | Yes | No | No |
| 17 | F/36/2550 | UVC, percutaneous | NoNo | Sepsis, erythrocytosisSteinert disease, | Cerebral sinuses | LMWH/14 | Yes | No | No |
| 18 | F/39/3290 | UVC, percutaneous | NoNo | congenital surfactant deficiency, left ventricular hypertrophy, right-side heart failureRDS, sepsis | Portal and umbilical | LMWH/11 | Yes, partial | No | No |
| 19 | M/34/2450 | UVC, UAC | No | Heart defect (transposition of great vessels), Rashkind atrial septostomyRDS | Right femoral vein | Watchful waiting, favourable outcome | Lost to follow-up | – | – |
| 20 | F/38/2600 | Percutaneous | No | – | Renal vein | Watchful waiting, progression of thrombosis | Yes | Renal atrophy | |
| 21 | F/36/3080 | UVC, percutaneous | NoNo | Dehydration,sepsis | Umbilical vein | Watchful waiting | Lost to follow-up | – | – |
| 22 | M/39/3040 | CVC | No | Oligoamnios, sepsis | Inferior vena cava | UFH→LMWH/1 | Yes | No | Renal atrophy |
| 23 | M/33/2990 | UVC | No | Maternal DM, preeclampsiaRDS, sepsis, corticoids | Right renal vein | Watchful waiting, coagulopathy | – | – | Renal failure, multipole organ failure, CA, death |
| 24 | M/37/2370 | UVC | No | Maternal DM, PROM,erythrocytosis | Umbilical vein | Watchful waiting,asymptomatic | Yes | – | No |
| 25 | M/31/1532 | UVC | No | Heart defect (ASD),RDS, erythrocytosis, sepsis,NEC, gastrointestinal surgery | Brachiocephalic and jugular arteries | LMWH/120 | No,collateral vessels | No | No |
| 26 | M/31/2320 | Percutaneous | No | Severe oligoamnios,RDS | Bilateral renal vein | LMWH/4 | Yes, partial,renal disease | No | Yes, grade 2 CKD grade II |
| 27 | M/37/2135 | UVC, percutaneous | NoYes | Sepsis, NEC, IUGR | Portal | LMWH/14 | Yes | No | Cavernomatosis and PHT |
| 28 | F/40/3190 | UVCUAC, percutaneous | Yes (portal)NoYes | PROM, asphyxia, hypothermia,RDS, sepsis | Portal | LMWH/7 | – | No | NoWithholding of treatment |
| 29 | F/31/1790 | UVC, percutaneous | NoNo | RDS, sepsis, erythrocytosis | Portal | Watchful waiting, coagulopathy | – | – | Refractory pulmonary haemorrhage -hypoxaemia, CA, death (familial intrahepatic cholestasis type 2) |
ASD, atrial septal defect; CA, cardiac arrest; CKD, chronic kidney disease; DM, diabetes mellitus; IUGR, intrauterine growth restriction; LMWH: low molecular weight heparin (150IU/kg/day; monitored by measurement of anti-factor Xa levels [target, 0.35–0.7IU/mL], first at 4 days and after weekly or every 15 days depending on measured levels); NEC, necrotising enterocolitis; NRFS, non-reassuring foetal status; PHT, pulmonary hypertension; PROM, premature rupture of membranes; rTPA, recombinant tissue plasminogen activator (0.1–0.5mg/kg/h twice daily for 7 days); PE, pulmonary embolism; RA, right atrium; RDS, respiratory distress syndrome; UAC, umbilical artery catheter; UFH, unfractionated heparin (14–28IU/kg/h); UVC, umbilical vein catheter.
A total of 6 patients died (20%): 2 (cases 11 and 13) as a direct result of thrombosis in the right atrium, with a clinical presentation compatible with pulmonary embolism. A third patient (case 23) died of hypoxic-ischaemic encephalopathy with multiple organ failure in the context of right renal vein thrombosis, with no other relevant findings in the autopsy. The remaining patients died of causes secondary to other diseases, such as pulmonary haemorrhage (case 29) or withholding of life-sustaining treatment in the context of other diseases (cases 15 and 28).
Of the 4 patients that had life- or organ-threatening thrombosis (cases 6, 10, 12 and 14), 2 died. Only 1 had a favourable outcome after treatment with bemiparin of a small atrial thrombus, which resolved in 8 weeks. The patient with bilateral renal artery thrombosis (case 6) was initially treated with fibrinolytic drugs, but the treatment had to be discontinued due to haemorrhage of the choroid plexus, after which she developed end-stage renal disease.
Five patients underwent an evaluation of thrombophilia, and abnormalities were found in 2 (factor V Leiden and factor II and XII, cases 6 and 26).
In the management of neonatal thrombosis, the morbidity and mortality are determined to a great extent by the location of the thrombus. The outcome depends on the optimal diagnosis and management, and therefore in patients at risk, if there is suspicion based on the clinical presentation or laboratory results (persistent thrombocytopenia), imaging tests should be requested at an early stage (Doppler ultrasound, CT angiogram or magnetic resonance angiogram), and an angiogram should be performed in cases with an uncertain diagnosis. We recommend against the D-dimer test in newborns.
The treatment of neonatal thrombosis poses dilemmas that are a source of controversy due to the risk of bleeding in this population, and there is considerable variability in its management between health providers and facilities. The clinical practice guidelines on antithrombotic therapy in newborns and children4 indicate that in cases of life- or organ-threatening thrombosis, and in the absence of absolute contraindications (surgery or central nervous system ischaemia, active bleeding, invasive procedures or seizures in the past 48–72h), initiation of thrombolytic therapy should be considered taking into account the size and location of the thrombus (such as: diameter>2cm and/or mobile right atrial thrombosis). The risk–benefit assessment should be individualised. There are different schemes for thrombolytic therapy with recombinant tissue plasminogen activator (rtPA), and at present there is no evidence supporting the superiority of any of them.4
If there is no risk of patient or organ death or thrombolytic therapy is contraindicated, treatment with an anticoagulant agent treatment should be initiated (low-molecular weight or unfractionated heparin)3 in patients who are symptomatic (hypertension, change in limb colour, persistent tachycardia, …), while in patients who are asymptomatic and in whom thrombosis was a chance finding, the decision whether to maintain a watchful waiting approach should be made on a case-by-case basis.
We recommend that the follow-up of patients with thrombosis, especially in cases with a related family history, of great severity (purpura fulminans) or in the absence of risk factors, include an investigation of thrombophilia.2
Although the reported evidence on the subject is limited, given the high morbidity and mortality associated with thrombosis in critical locations (50% of our sample) and that the reviewed literature offers encouraging data regarding the use of fibrinolytic agents (even in patients born preterm),5,6 early use of thrombolytic therapy should be considered in life- or organ-threatening cases as long as the hospital has the necessary resources and experienced staff, always with an individualised risk–benefit assessment.
Our study was retrospective, and it is important to take into account the limitations intrinsic to this type of design.
Please cite this article as: Alonso Montejo MdM, Artacho González L, Serrano Martín MdM. Trombosis en cuidados críticos neonatales: nuestra experiencia en 10 años. An Pediatr (Barc). 2019;91:47–53.