Despite there being limited evidence, non-invasive ventilation (NIV) has become a common treatment for acute respiratory failure (ARF). The aim of this study was to identify the predictive factors of NIV failure, in order to enable early detection of patients failing the treatment.
Patients and methodsProspective cohort study was conducted that included all ARF patients that received NIV as the initial treatment between 2005 and 2009 in a fourteen-bed Paediatric Intensive Care Unit (PICU) of a tertiary university hospital. Information was collected about the NIV, as well as clinical data prior to NIV, at 2, 8, 12, and 24h. The haemoglobin saturation (SpO2)/fraction of inspired oxygen (FiO2) ratio (S/F) was retrospectively calculated. NIV failure was defined as the need for intubation or requiring rescue with bi-level pressure (BLPAP). Univariate and multivariate statistical analyses were performed.
ResultsA total of 282 patients received non-invasive support, with 71 receiving Continuous Pressure (CPAP), and 211 with BLPAP treatment. The overall success rate was 71%. Patients receiving BLPAP vs. CPAP, patients with higher S/F ratios at 2h (odds ratio [OR] 0.991, 95% CI: 0.986–0.996, P=.001), and patients older than 6 months (Hazard ratio [HZ] 0.375, 95% CI: 0.171–0.820, P=.014), were also more likely to fail. Patients with higher heart rates (HR) at 2h (OR 1.021, 95% CI [1.008–1.034], P=.001) and higher inspiratory positive airway pressure (IPAP) at 2h were more prone to failure (HZ 1.214, 95% CI [1.046–1.408], P=.011).
ConclusionsAge below 6 months, S/F ratio, HR, and IPAP at 2h are independent predictive factors for initial NIV failure in paediatric patients with ARF admitted to the PICU.
La ventilación no invasiva (VNI) se ha convertido en un tratamiento habitual de la insuficiencia respiratoria aguda (IRA). Nuestro objetivo ha sido identificar factores predictores de fracaso de VNI para detectar precozmente a los pacientes en los que no tendrá éxito.
Pacientes y métodosEstudio de cohortes prospectivo que incluyó a todos los pacientes con IRA que recibieron VNI como tratamiento inicial entre 2005 y 2009, en una unidad de cuidados intensivos pediátricos de 14 camas de un hospital universitario de tercer nivel. Se recogieron datos clínicos e información sobre la VNI, previamente a su inicio, a las 2, 8, 12 y 24horas. La razón entre saturación de hemoglobina y fracción de oxígeno inspirada (S/F) se calculó retrospectivamente. Se definió fallo de VNI como necesidad de intubación o necesidad de rescate con presión binivel (BLPAP). Se realizaron análisis estadísticos univariable y multivariable.
ResultadosUn total de n=282 pacientes recibieron soporte no invasivo, presión continua=71, BLPAP=211. El porcentaje de éxito de la muestra global fue 71%. Los pacientes tratados con BLPAP vs. presión continua, aquellos con S/F más elevados a las 2horas (odds ratio 0,991, IC 95%: 0,986-0,996, p=0,001) y los mayores de 6 meses (hazard ratio 0,375, IC 95% 0,171-0,820, p=0,014), presentaron menor riesgo de fracaso. Los pacientes con frecuencias cardíacas más altas y mayor presión positiva inspiratoria en vía aérea a las 2horas (odds ratio 1,021, IC 95%: 1,008-1,034, p=0,001; hazard ratio 1,214, IC 95%: 1,046-1,408, p=0,011) presentaron mayor riesgo de fracaso.
ConclusionesLa edad<6 meses, S/F, frecuencia cardíaca y presión positiva inspiratoria en la vía aérea a las 2horas son factores predictores independientes de fracaso de VNI inicial en pacientes con IRA admitidos en una unidad de cuidados intensivos pediátricos.
Non-invasive ventilation (NIV) has become a widely accepted therapy for management of acute respiratory failure (ARF) in paediatric patients. To date, only one randomised controlled trial has been performed in the paediatric population in patients admitted to a single paediatric intensive care unit (PICU),1 which found a reduction in the proportion of patients that required endotracheal intubation in the group managed with NIV. However, there is evidence that maintenance of NIV in adult patients that do not respond to this treatment increases the risk of complications and even death.2 Thus, it is essential to identify predictors for NIV failure to allow the early identification of patients in whom NIV will fail in order to avoid prolonging an inappropriate treatment. Some of the risk factors identified to date are hypoxaemia,3–6 acute respiratory distress syndrome,7 a high score in severity scales (PELOD, PRISM II),6–10 work of breathing,1,6,10,11 age,4,10 hypercapnia,4,7–9 blood pressure12 and the use of nasal prongs or ventilators without an air/oxygen blender in hypoxic patients.13
The primary objective of our study was to identify predictors for failure of initial NIV in the PICU patient population. The secondary objective was to describe the characteristics of NIV as first-line treatment in children with ARF.
MethodsPatients and settingWe conducted a prospective cohort study in the 14-bed PICU of a tertiary care hospital between January 2005 and December 2009. We used consecutive sampling to recruit patients aged 0–18 years with a diagnosis of ARF admitted to the PICU that received NIV as initial treatment. Non-invasive ventilation was initiated in patients that met the clinical criteria for ARF and at high probability of intubation in the next 6h. This decision was made by the clinician in charge of the patient if the patient presented any of the following signs: respiratory rate (RR) more than 2 standard deviations (SDs) above normal, need of a fraction of inspired oxygen (FiO2) greater than 0.5 to maintain the percentage of oxygenated haemoglobin (peripheral capillary oxygen saturation, SpO2) above 94% or progressive dyspnoea at rest. We categorised respiratory failure as type I (changes in ventilation/perfusion without impairment of gas exchange at the alveolar level) or type II (changes accompanied by impaired gas exchange in alveoli) based on the classification proposed by Newth.14 The exclusion criteria were the presence of any contraindication for the use of NIV15: cardiac arrest, haemodynamic instability requiring inotropic therapy, severe arrhythmia, Glasgow coma scale score of less than 10, facial trauma, surgery, vocal cord paralysis, undrained pneumothorax and active upper gastrointestinal bleeding. We also excluded patients that were intubated or ventilated through a tracheostomy tube at the time of admission, as well as patients that received NIV after extubation or palliative NIV (such as patients with diseases where intubation is considered a futile intervention). The study was approved by the Ethics and Research Committee of the Hospital Sant Joan de Déu and considered exempt of the need for informed consent, as NIV is a routine treatment in the PICU and consent for the use of this type of data was not necessary at the time of the study.
Non-invasive ventilation techniqueThe physician in charge of the patient was responsible for the decision to choose between continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BiPAP). Continuous positive airway pressure was used in patients with apnoea or mild dyspnoea, and BiPAP in patients with moderate to severe dyspnoea, significantly increased work of breathing (based on lung sounds and diaphragmatic excursion) despite the use of CPAP, low inspiratory capacity or progressive hypercapnia.
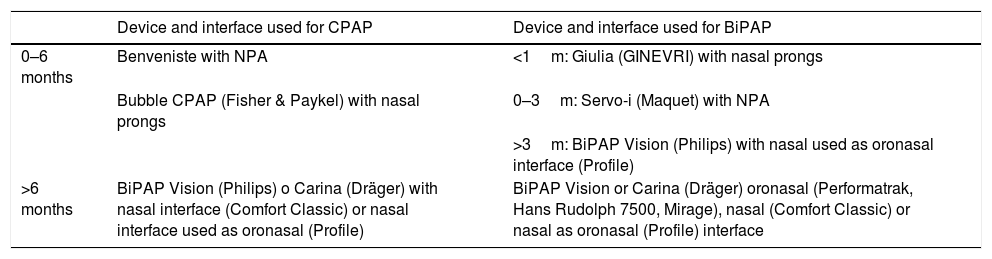
MaterialsWe used the following interfaces: nasal prongs for the Bubble CPAP system (Fisher & Paykel Healthcare Ltd., New Zealand) and the Giulia™ ventilator (GINEVRI, Italy); Profile Lite™ and Comfortclassic™ Philips Respironics nasal masks (Philips Respironics, Netherlands) used as oronasal masks; the Mirage™ (ResMed, California, USA), PerformaTrak® (Philips Respironics, Netherlands) and 7500® (Hans Rudolph Inc., Kansas, USA) oronasal masks; and endotracheal tubes used as nasopharyngeal tubes. Table 1 details the criteria used to select the interface and ventilator. Comfeel™ hydrocolloid dressings (Coloplast, Denmark) and Mepentol™ hyperoxygenated fatty acid solution (Bama-Geve, Spain) were used to prevent pressure ulcers. Active humidification (Fisher & Paykel) was used in every patient starting in 2007.
Criteria used to select the interface and ventilator.
| Device and interface used for CPAP | Device and interface used for BiPAP | |
|---|---|---|
| 0–6 months | Benveniste with NPA | <1m: Giulia (GINEVRI) with nasal prongs |
| Bubble CPAP (Fisher & Paykel) with nasal prongs | 0–3m: Servo-i (Maquet) with NPA | |
| >3m: BiPAP Vision (Philips) with nasal used as oronasal interface (Profile) | ||
| >6 months | BiPAP Vision (Philips) o Carina (Dräger) with nasal interface (Comfort Classic) or nasal interface used as oronasal (Profile) | BiPAP Vision or Carina (Dräger) oronasal (Performatrak, Hans Rudolph 7500, Mirage), nasal (Comfort Classic) or nasal as oronasal (Profile) interface |
NPA, nasopharyngeal airway.
The Benveniste valve (used in our unit in 64% of patients aged 0–6 months) is an adaptation crafted by hand of a water seal connected to the interface (usually a NPA) by means of an open ring that helps reduce the work of breathing in the expiratory phase.
The ventilation strategy was selected according to the protocol established by the Working Group on Respiratory Care of the Sociedad Española de Cuidados Intensivos Pediátricos (Spanish Society of Paediatric Intensive Care).15 The initial pressure set for CPAP was 4–5cmH2O. In patients that did not experience improvement in work of breathing or apnoea, the pressure was increased to up to 8cmH2O. The initial settings for BiPAP were an inspiratory positive airway pressure (IPAP) or maximum inspiratory pressure of 6–8cmH2O and an expiratory positive airway pressure (EPAP) or positive pressure at the end of expiration of 4cmH2O. The IPAP was increased in 2cmH2O increments to up to 22cmH2O in case of an inadequate tidal volume, insufficient improvement of work of breathing or hypercapnia. The EPAP was increased to up to 8cmH2O to improve alveolar recruitment and oxygenation. There was no set maximum FiO2.
If any sign of treatment failure was detected, NIV was discontinued and the patient underwent intubation and started conventional mechanical ventilation. The following were considered signs of failure: SpO2<85% and a partial pressure of carbon dioxide (pCO2) greater than 65mmHg despite application of maximum settings in NIV, increase in work of breathing, apnoea or development of exclusion criteria. Patients were weaned of NIV gradually, with stepwise decreases in IPAP by 2cmH2O and in EPAP by 1cmH2O when the patient experienced a decrease in RR and work of breathing and the FiO2 was less than 0.4. The patients underwent placement of a nasogastric tube to avoid abdominal distension and for administration of enteral feedings.
MonitoringAll patients underwent continuous monitoring of heart rate, RR and SpO2. Peripheral venous blood gas analysis was only performed when the physician in charge considered it necessary.
SedationWhen nonpharmacological measures were insufficient to prevent patient-ventilator asynchrony, the patient received sedatives (in monotherapy or as combination therapy), such as oral levomepromazine (1mg/kg/dose), continuous intravenous infusion of midazolam (0.05–0.1mg/kg/h) or propofol (1–2mg/kg/h) for less than 24h.
Data collectionFor each episode, we recorded the age and sex of the patient, the underlying disease and the severity score (Paediatric Risk of Mortality Score II [PRISM II]). We also collected data on variables related to NIV: aetiology of ARF, type of respiratory failure, mode of ventilation, ventilatory parameters (FiO2, IPAP, EPAP), physiological variables (HR, RR and SpO2) prior to initiation of NIV and at 2, 8, 12 and 24h of treatment, changes (numerical difference in values) in HR and RR relative to baseline, peripheral venous blood gas values (when obtained), changes in the mode of ventilation, ventilator or interfaces used, administration of sedatives, development of complications or contraindications, mortality, duration of NIV, outcome of NIV (success/failure) and length of stay in the PICU.
We defined NIV failure as the need for intubation or rescue with BiPAP in the group initially treated with CPAP. The very strong correlation between the SaO2/FiO2 (S/F) and PaO2/FiO2 (P/F) ratios was confirmed in the paediatric population in 2009.16 Therefore, we calculated the S/F ratio retrospectively and assessed its potential usefulness as a predictor of NIV failure. We calculated the S/F ratio for the baseline before initiation of NIV and at 2, 4, 8, 12 and 24 of NIV, excluding any values of SpO2 greater than 97%.
Statistical analysisWe performed a descriptive analysis, expressing qualitative variables as percentages and 95% confidence intervals (CIs) and quantitative variables as mean or median as a measure of central tendency, and standard deviation or interquartile range (IQR) as a measure of dispersion, depending on whether or not the data distribution was normal. We assessed the correlation of outcome measures with different variables under study by means of the chi square test, the Student t test or the Mann–Whitney U test, depending on the shape of the distribution. We performed a multivariate analysis fitting forward stepwise binary logistic regression and Cox regression models to identify potential predictors of NIV success or failure, calculating the odds ratios and hazard ratios, respectively, with their corresponding 95% CIs. The variables selected for the model were those corresponding to p-values of less than 0.1 in the univariate analysis or which had been found to be significant in the previous literature. In case of potential colinearity, we selected the variable for which there was data earlier in time. We provide a measure of the predictive power of the model, the −2 log-likelihood statistic (−2LL), which is better the lower the value of the statistic, to better inform the reader.
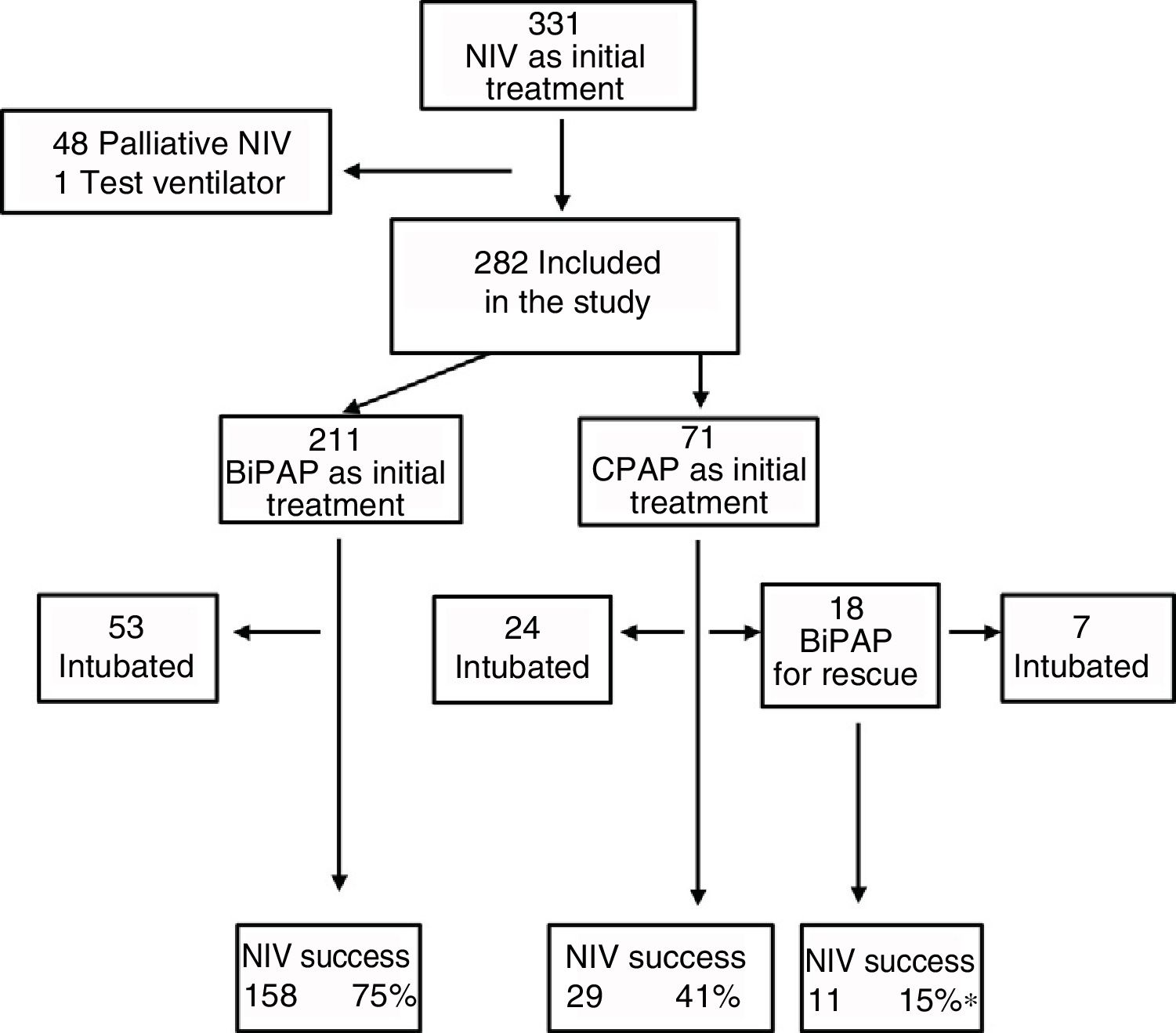
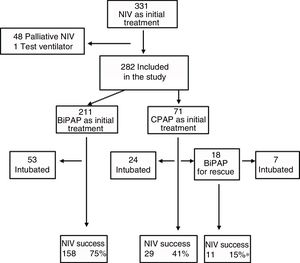
ResultsDescriptive analysisIn the period under study, 5101 patients were admitted to the PICU, of who 2238 (43.9%) required ventilatory support. Within this subset, 331 (14.8%) received NIV as the initial mode of ventilation. We excluded 48 patients that received NIV for palliative care and 1 case where a test ventilator was used. Thus, the final sample comprised 282 patients (12.6%), of who 71 were managed with CPAP and the rest with BiPAP (Fig. 1). In the CPAP group, 14% of patients had type I respiratory failure, compared to 40% in the BiPAP group.
The most frequent relevant medical conditions in the sample were congenital heart disease (12.4%), chronic neuromuscular disease (9.1%), cardiovascular postoperative period (8.7%), history of preterm birth (3.2%) and Down syndrome (2%); 47% of the patients were previously healthy. The diagnoses associated with ARF most frequently were bronchiolitis (31%), pneumonia (15%), viral respiratory infection (9%) and asthma (7%).
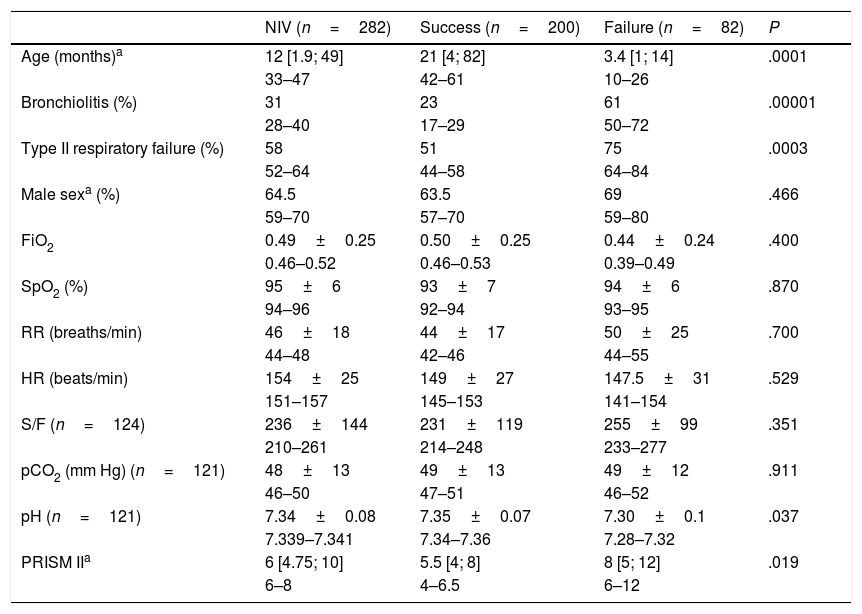
Sedation was used in 47% of cases to promote patient-ventilator synchronisation, and oral levomepromazine was the drug used most frequently for the purpose. Fifteen patients required a change of interface due to leaks. Complications were mild and infrequent: pressure ulcers (n=11), pneumomediastinum (n=1), gastric distention (n=1), hypercapnia (n=1) and conjunctivitis (n=1). Four patients died, and none of these deaths could be attributed to the previous use of NIV. The proportion of success in our study was 71%, although there were differences based on the initial treatment, with the lowest proportion (55%) in the group treated exclusively with CPAP (Fig. 1). As Fig. 1 shows, 18 patients required rescue with BiPAP, which succeeded in preventing intubation in 11 (61%). Table 2 presents the baseline data for the total sample comparing the NIV success and NIV failure subsets. We found significant differences between the success and failure subsets at baseline for age (21 vs. 3.4 months), blood pH, type of respiratory failure and frequency of bronchiolitis diagnosis, and in the PRISM score at 24h (5.5 vs. 8).
Baseline characteristics of patients by success or failure of NIV.
| NIV (n=282) | Success (n=200) | Failure (n=82) | P | |
|---|---|---|---|---|
| Age (months)a | 12 [1.9; 49] | 21 [4; 82] | 3.4 [1; 14] | .0001 |
| 33–47 | 42–61 | 10–26 | ||
| Bronchiolitis (%) | 31 | 23 | 61 | .00001 |
| 28–40 | 17–29 | 50–72 | ||
| Type II respiratory failure (%) | 58 | 51 | 75 | .0003 |
| 52–64 | 44–58 | 64–84 | ||
| Male sexa (%) | 64.5 | 63.5 | 69 | .466 |
| 59–70 | 57–70 | 59–80 | ||
| FiO2 | 0.49±0.25 | 0.50±0.25 | 0.44±0.24 | .400 |
| 0.46–0.52 | 0.46–0.53 | 0.39–0.49 | ||
| SpO2 (%) | 95±6 | 93±7 | 94±6 | .870 |
| 94–96 | 92–94 | 93–95 | ||
| RR (breaths/min) | 46±18 | 44±17 | 50±25 | .700 |
| 44–48 | 42–46 | 44–55 | ||
| HR (beats/min) | 154±25 | 149±27 | 147.5±31 | .529 |
| 151–157 | 145–153 | 141–154 | ||
| S/F (n=124) | 236±144 | 231±119 | 255±99 | .351 |
| 210–261 | 214–248 | 233–277 | ||
| pCO2 (mm Hg) (n=121) | 48±13 | 49±13 | 49±12 | .911 |
| 46–50 | 47–51 | 46–52 | ||
| pH (n=121) | 7.34±0.08 | 7.35±0.07 | 7.30±0.1 | .037 |
| 7.339–7.341 | 7.34–7.36 | 7.28–7.32 | ||
| PRISM IIa | 6 [4.75; 10] | 5.5 [4; 8] | 8 [5; 12] | .019 |
| 6–8 | 4–6.5 | 6–12 |
FiO2, fraction of inspired oxygen; HR, heart rate; pCO2, partial pressure of carbon dioxide; PRISM II, Paediatric Risk Score of Mortality II; RR, respiratory rate; S/F, haemoglobin saturation/fraction of inspired oxygen; SpO2, haemoglobin saturation.
The age and PRISM II, whose distribution was not normal, are expressed as median and interquartile range; sex, presence of bronchiolitis and presence of type II respiratory failure as absolute frequencies and percentages, and the rest of the variables, which followed a normal distribution, as mean±standard deviation. For all these statistics, the 95% confidence interval for the sample is given below the value.
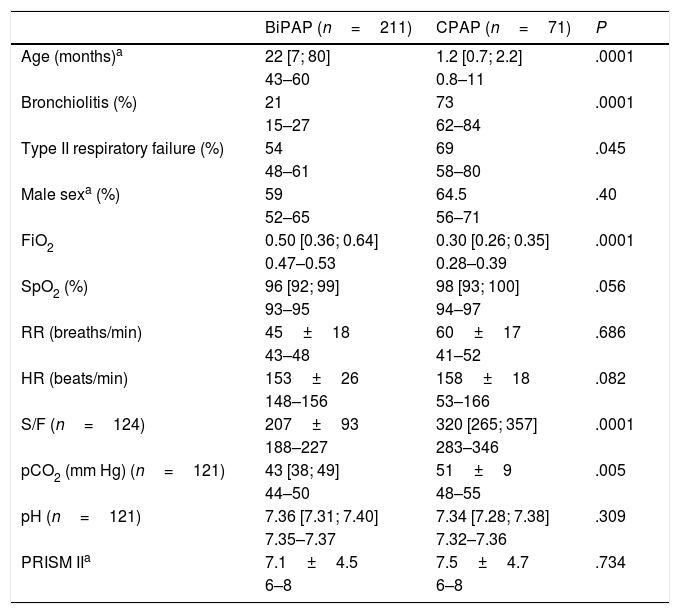
Table 3 compares the patients treated with CPAP versus BiPAP, highlighting that patients that received CPAP were significantly younger and had lower oxygen requirements compared to patients treated with BiPAP.
Characteristics of patients before initiation of NIV. Comparison of CPAP and BiPAP modes.
| BiPAP (n=211) | CPAP (n=71) | P | |
|---|---|---|---|
| Age (months)a | 22 [7; 80] | 1.2 [0.7; 2.2] | .0001 |
| 43–60 | 0.8–11 | ||
| Bronchiolitis (%) | 21 | 73 | .0001 |
| 15–27 | 62–84 | ||
| Type II respiratory failure (%) | 54 | 69 | .045 |
| 48–61 | 58–80 | ||
| Male sexa (%) | 59 | 64.5 | .40 |
| 52–65 | 56–71 | ||
| FiO2 | 0.50 [0.36; 0.64] | 0.30 [0.26; 0.35] | .0001 |
| 0.47–0.53 | 0.28–0.39 | ||
| SpO2 (%) | 96 [92; 99] | 98 [93; 100] | .056 |
| 93–95 | 94–97 | ||
| RR (breaths/min) | 45±18 | 60±17 | .686 |
| 43–48 | 41–52 | ||
| HR (beats/min) | 153±26 | 158±18 | .082 |
| 148–156 | 53–166 | ||
| S/F (n=124) | 207±93 | 320 [265; 357] | .0001 |
| 188–227 | 283–346 | ||
| pCO2 (mm Hg) (n=121) | 43 [38; 49] | 51±9 | .005 |
| 44–50 | 48–55 | ||
| pH (n=121) | 7.36 [7.31; 7.40] | 7.34 [7.28; 7.38] | .309 |
| 7.35–7.37 | 7.32–7.36 | ||
| PRISM IIa | 7.1±4.5 | 7.5±4.7 | .734 |
| 6–8 | 6–8 |
HR, heart rate; FiO2, fraction of inspired oxygen; pCO2, partial pressure of carbon dioxide; PRISM II, Paediatric Risk Score Mortality II; RR, respiratory rate; S/F, haemoglobin saturation/fraction of inspired oxygen; SpO2, haemoglobin saturation.
Age, the PRISM II score and other variables that did not have a normal distribution are expressed as median and interquartile range; sex as percentages, and the rest of the variables, which met the normality assumption, as mean±standard deviation. For all these statistics, the 95% confidence interval for the sample is given below the value.
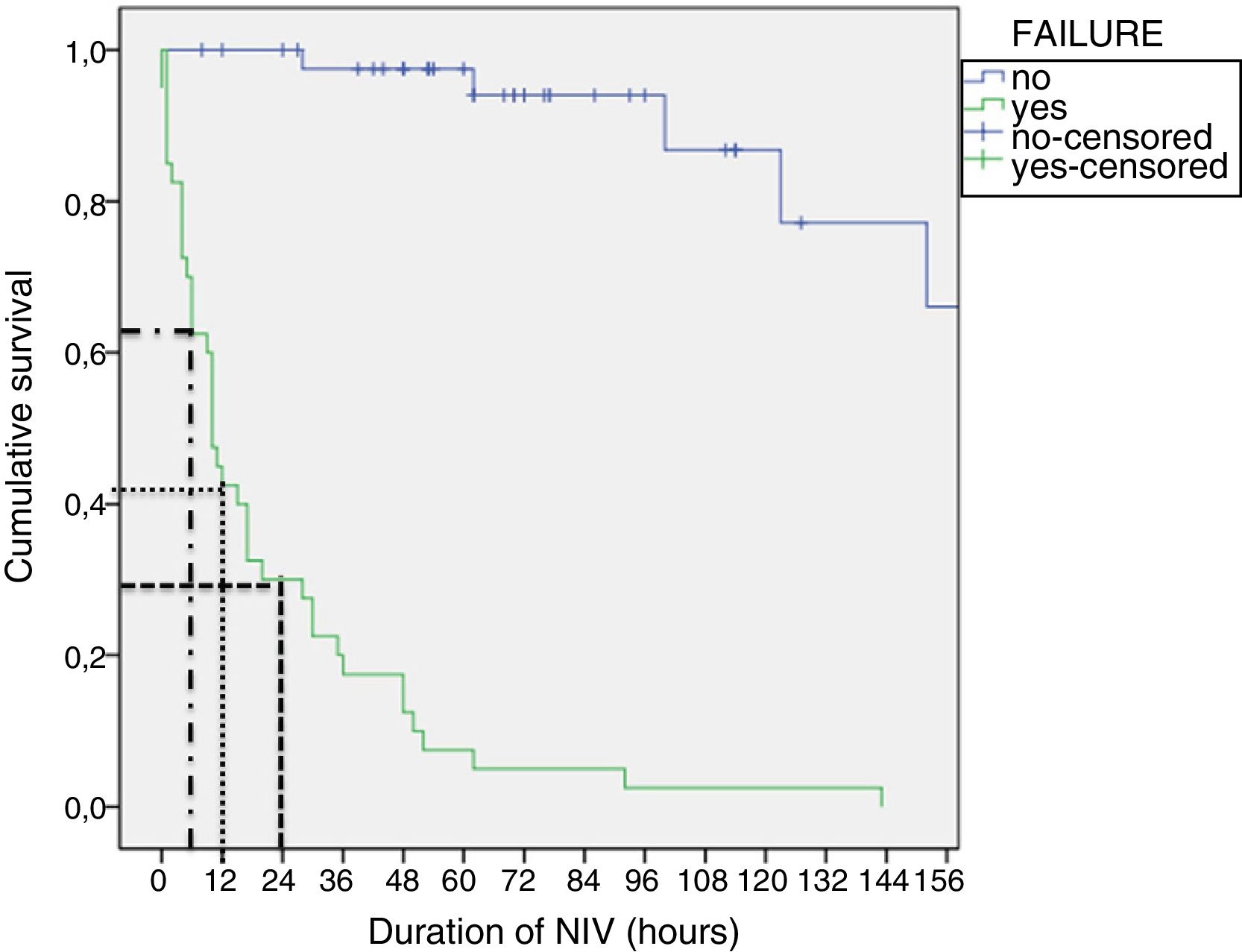
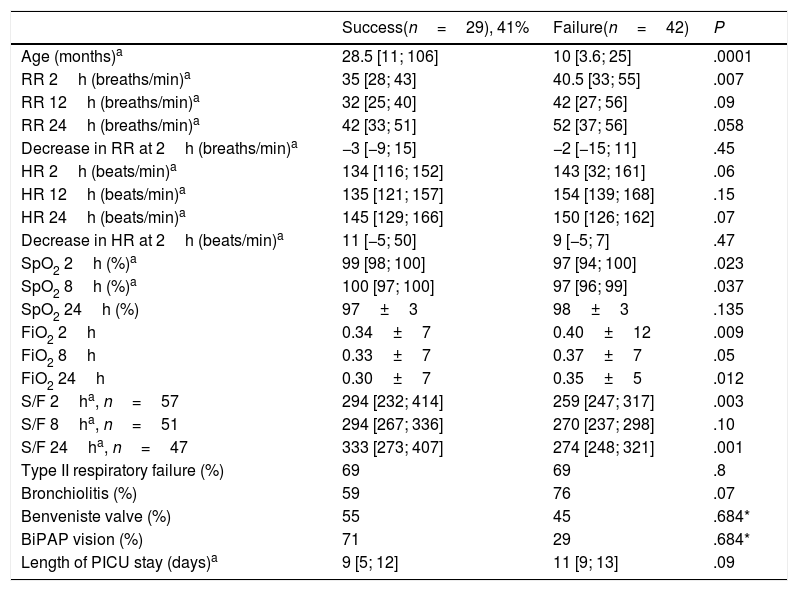
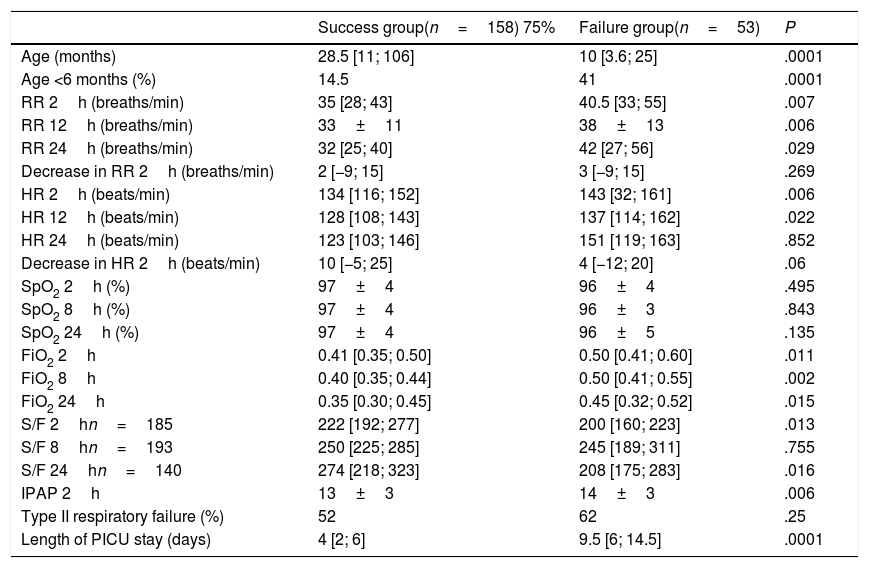
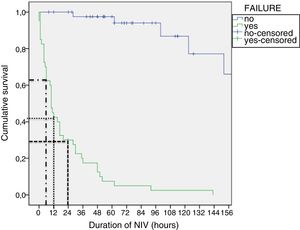
Tables 4 and 5 present the differences between the NIV success and NIV failure subsets of patients in the CPAP group and the BiPAP group, respectively. Although we ought to highlight the difference in efficacy between NIV devices (Benveniste, 55%; BiPAP Vision® 71%) it was not statistically significant. In the BiPAP group, patients in whom NIV failed were significantly younger (10 vs. 28.5 months, P=.001), had significantly higher RRs (51 vs. 41bpm, P=.010) and had significantly lower blood pH values (pH 7.30 vs. 7.35, P=.014) prior to initiation of NIV compared to patients that did not require intubation. As can be seen in Fig. 2, approximately 70% of patients were intubated in the first 24h. The most frequent causes of intubation were the presence of clinical signs of respiratory muscle fatigue (absence of improvement in HR and RR, thoracoabdominal asynchrony) and persistent hypoxaemia.
Study variables in the group treated with CPAP based on the success or failure of NIV.
| Success(n=29), 41% | Failure(n=42) | P | |
|---|---|---|---|
| Age (months)a | 28.5 [11; 106] | 10 [3.6; 25] | .0001 |
| RR 2h (breaths/min)a | 35 [28; 43] | 40.5 [33; 55] | .007 |
| RR 12h (breaths/min)a | 32 [25; 40] | 42 [27; 56] | .09 |
| RR 24h (breaths/min)a | 42 [33; 51] | 52 [37; 56] | .058 |
| Decrease in RR at 2h (breaths/min)a | −3 [−9; 15] | −2 [−15; 11] | .45 |
| HR 2h (beats/min)a | 134 [116; 152] | 143 [32; 161] | .06 |
| HR 12h (beats/min)a | 135 [121; 157] | 154 [139; 168] | .15 |
| HR 24h (beats/min)a | 145 [129; 166] | 150 [126; 162] | .07 |
| Decrease in HR at 2h (beats/min)a | 11 [−5; 50] | 9 [−5; 7] | .47 |
| SpO2 2h (%)a | 99 [98; 100] | 97 [94; 100] | .023 |
| SpO2 8h (%)a | 100 [97; 100] | 97 [96; 99] | .037 |
| SpO2 24h (%) | 97±3 | 98±3 | .135 |
| FiO2 2h | 0.34±7 | 0.40±12 | .009 |
| FiO2 8h | 0.33±7 | 0.37±7 | .05 |
| FiO2 24h | 0.30±7 | 0.35±5 | .012 |
| S/F 2ha, n=57 | 294 [232; 414] | 259 [247; 317] | .003 |
| S/F 8ha, n=51 | 294 [267; 336] | 270 [237; 298] | .10 |
| S/F 24ha, n=47 | 333 [273; 407] | 274 [248; 321] | .001 |
| Type II respiratory failure (%) | 69 | 69 | .8 |
| Bronchiolitis (%) | 59 | 76 | .07 |
| Benveniste valve (%) | 55 | 45 | .684* |
| BiPAP vision (%) | 71 | 29 | .684* |
| Length of PICU stay (days)a | 9 [5; 12] | 11 [9; 13] | .09 |
FiO2, fraction of inspired oxygen; HR, heart rate; RR, respiratory rate; S/F, haemoglobin saturation/fraction of inspired oxygen; SpO2, haemoglobin saturation.
Study variables in the group treated with bilevel positive airway pressure based on the success or failure of NIV.
| Success group(n=158) 75% | Failure group(n=53) | P | |
|---|---|---|---|
| Age (months) | 28.5 [11; 106] | 10 [3.6; 25] | .0001 |
| Age <6 months (%) | 14.5 | 41 | .0001 |
| RR 2h (breaths/min) | 35 [28; 43] | 40.5 [33; 55] | .007 |
| RR 12h (breaths/min) | 33±11 | 38±13 | .006 |
| RR 24h (breaths/min) | 32 [25; 40] | 42 [27; 56] | .029 |
| Decrease in RR 2h (breaths/min) | 2 [−9; 15] | 3 [−9; 15] | .269 |
| HR 2h (beats/min) | 134 [116; 152] | 143 [32; 161] | .006 |
| HR 12h (beats/min) | 128 [108; 143] | 137 [114; 162] | .022 |
| HR 24h (beats/min) | 123 [103; 146] | 151 [119; 163] | .852 |
| Decrease in HR 2h (beats/min) | 10 [−5; 25] | 4 [−12; 20] | .06 |
| SpO2 2h (%) | 97±4 | 96±4 | .495 |
| SpO2 8h (%) | 97±4 | 96±3 | .843 |
| SpO2 24h (%) | 97±4 | 96±5 | .135 |
| FiO2 2h | 0.41 [0.35; 0.50] | 0.50 [0.41; 0.60] | .011 |
| FiO2 8h | 0.40 [0.35; 0.44] | 0.50 [0.41; 0.55] | .002 |
| FiO2 24h | 0.35 [0.30; 0.45] | 0.45 [0.32; 0.52] | .015 |
| S/F 2hn=185 | 222 [192; 277] | 200 [160; 223] | .013 |
| S/F 8hn=193 | 250 [225; 285] | 245 [189; 311] | .755 |
| S/F 24hn=140 | 274 [218; 323] | 208 [175; 283] | .016 |
| IPAP 2h | 13±3 | 14±3 | .006 |
| Type II respiratory failure (%) | 52 | 62 | .25 |
| Length of PICU stay (days) | 4 [2; 6] | 9.5 [6; 14.5] | .0001 |
HR, heart rate; FiO2, fraction of inspired oxygen; h, hours/hours of treatment with NIV; IPAP, inspiratory positive airway pressure; PICU, paediatric intensive care unit; RR, respiratory rate; S/F, haemoglobin saturation/fraction of inspired oxygen; SpO2, haemoglobin saturation.
Lastly, the length of stay in the PICU was significantly longer in patients that experienced BiPAP failure.
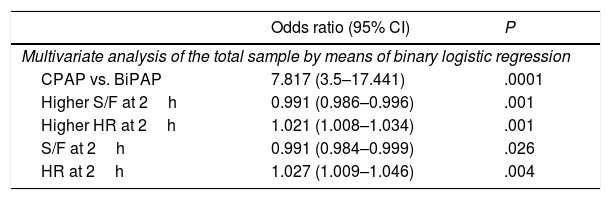
Multivariate analysis (Table 6)We performed a multivariate analysis of the data for the total sample by means of binary logistic regression (BLR). We analysed the following variables: mode of ventilation, type of respiratory failure, age 6 months (greater than/less than), RR, HR and S/F ration at 2h of treatment and bronchiolitis (yes/no) (predictive power, −2LL=232). We also performed a multivariate analysis of the subset of patients that received BiPAP at some point during the course of disease by means of Cox regression. We analysed the following variables: mode of ventilation age 6 months (greater than/less than) and HR, S/F ad IPAP at 2h. Good predictive power (−2LL=22).
Summary of the multivariate analysis.
| Odds ratio (95% CI) | P | |
|---|---|---|
| Multivariate analysis of the total sample by means of binary logistic regression | ||
| CPAP vs. BiPAP | 7.817 (3.5–17.441) | .0001 |
| Higher S/F at 2h | 0.991 (0.986–0.996) | .001 |
| Higher HR at 2h | 1.021 (1.008–1.034) | .001 |
| S/F at 2h | 0.991 (0.984–0.999) | .026 |
| HR at 2h | 1.027 (1.009–1.046) | .004 |
| Hazard ratio (95% CI) | P | |
|---|---|---|
| Multivariate analysis of the patient subset managed with BiPAP at some point during their stay by means of Cox regression | ||
| Age greater/less than 6 months | 0.375 (0.171–0.820) | .014 |
| IPAP mayor at 2h | 1.214 (1.046–1.408) | .011 |
| Multivariate analysis of the patient subset treated exclusively with BiPAP by means of Cox regression | ||
| S/F at 2h | 0.991 (0.984–0.999) | .026 |
| HR at 2h | 1.024 (1.008–1.040) | .003 |
| IPAP at 2h | 1.030 (1.030–1.351) | .017 |
Another multivariate analysis involved the group treated exclusively with BiPAP.
- -
BLR: the variables analysed were age 6 months (greater than/less than), values of IPAP, S/F ratio and HR and the decrease in the RR at 2h. Good predictive power (−2LL=131).
- -
Cox regression analysis: the variables analysed were age 6 months (greater than/less than), values of IPAP, HR and S/F ratio at 2h. Lesser predictive power (−2LL=240).
Although we also performed a multivariate analysis in the CPAP group, we did not find statistically significant differences due to the small size of this subset of patients.
DiscussionOur analysis identified 2 important predictors of NIV failure. The first and most strongly correlated was age, which is consistent with previous studies.4,11 More specifically, we found that age less than 6 months predicted NIV failure. This could be explained by the anatomical and physiological characteristics of this age group, which would result in a higher incidence of severe respiratory failure. Furthermore, these children have apnoeic episodes more frequently,17 and those born preterm in particular are more likely to experience patient-ventilator asynchrony18 compared to patients aged more than 7 months.19 On the other hand, the technical limitations of the ventilators and interfaces used in this age group complicate the use of NIV.
Another important predictor identified in the total sample was initial use of CPAP, which contradicts the findings of James et al.12 Since severe apnoea was not the reason for NIV failure in patients treated with CPAP with the exception of 2 cases, this difference could be due to the use of the Benveniste valve in most patients in the CPAP group, a device that is less effective (and is no longer used in our unit) because it does not allow measurement of pressures or volumes or compensating for leaks. On the other hand, the use of conventional ventilators in younger children,3 as was done in the study by James et al, has been shown to have poorer outcomes compared to the use of ventilators specifically designed for NIV.6,10,20 Thus, both their and our study probably reflect the technical limitations of the devices used, as opposed to whether the mode of ventilation is an actual predictor.21
The S/F ratio performed as a predictor of NIV failure, especially the lowest values at 2h of treatment. The largest study conducted to date in the paediatric population (390 episodes) that used the S/F ratio as a monitoring measure demonstrated its validity as a predictor of failure at 1h and 6h of treatment,22 and the authors proposed to cut-off point of 200 for this ratio. Along these lines, previous studies had already demonstrated that hypoxaemia was a predictor of failure, with the authors proposing a P/F ratio of less than 175 in adults23 and an S/F ratio of less than 189 in children24 for indication of intubation. Based on the cumulative evidence to date, the S/F ratio could be used as a standardised non-invasive marker of oxygen saturation in patients in whom vascular access cannot be established, a situation that is frequent in paediatric patients requiring NIV.
When it came to other variables that are indirect measures of the work of breathing, we also found that higher values of the HR at 2h of treatment independently predicted failure, a result that was consistent with previous reports,1,11 a reminder of the importance of closely monitoring HR, especially at the beginning of treatment. Our study also identified high values of the IPAP at 2h as an independent predictor of failure. This agreed with the findings of a previous study5 that identified a mean airway pressure (MAP) greater than 11.5cmH2O in conventional ventilators as a predictor of failure. Since the MAP cannot be measured by ventilators specifically designed for NIV, the IPAP, equivalent to the peak pressure, could be a more useful parameter, as it is measured by all ventilators.
We ought to highlight that the odds ratios and hazard ratios corresponding to the variables HR, S/F ratio and IPAP at 2h had values close to 1, so that any of them in isolation cannot guide clinical decision-making. Our findings are consistent with the current data and suggest that the early identification of patients at risk of NIV failure must be based on age and the monitoring of hypoxaemia and work of breathing. While previous studies have identified the PRISM II as a predictor,6–10 we ruled out its use in our study, as this score is obtained at least 24h after admission, and a large proportion of patients managed with NIV require intubation before this time, which makes this score useless for predicting failure in the first 24h.
There are several limitations to our study. First of all, our decision to define failure of CPAP not only as endotracheal intubation but also as the need of rescue with BiPAP may be confusing to the reader and be a source of bias through uncontrolled confounders in the multivariate analysis. Another potential weakness is the loss of patients with SpO2 values of more than 97% in the multivariate analysis, which reduced the power of the S/F ratio as treatment with NIV progressed. However, substituting the FiO2 (n=282) for the S/F ratio in this Cox regression model lessened the predictive power (increase in the −2LL), which reinforced the validity of the data obtained using the S/F ratio. Since peripheral venous blood gas analysis was not routinely performed in every patient before and during NIV, our study was also severely limited in the analysis of monitoring of acidosis/hypercapnia and for the purpose of comparison with other studies.
Lastly, the considerable diversity in the equipment required based on the age of the patient also limits the generalisation of our findings to other hospitals, especially when it comes to the delivery of CPAP with a device handcrafted at the hospital.
Given the introduction of multiple devices for delivery of CPAP and BiPAP, of new modes of ventilation such as neurally adjusted ventilatory assist (NAVA) or interfaces like the full-face mask, and the fact that the data of our study were collected at least 9 years ago, our conclusions may be debatable. However, there are many facilities where these innovations have not been introduced, so our results may still be considered valid and pertinent in those facilities where there have been no substantial changes in the equipment or protocols used.
ConclusionsOur study found that lower S/F ratios and higher HR values at 2h of treatment were independently associated with an increased risk of failure of NIV both in the total sample and in the group treated with BiBAP, although this increase was minimal. In the group of patients managed with BiPAP at some point during their stay, we also found that a higher inspiratory pressure at 2h of treatment and age less than 6 months were associated with an increased risk of endotracheal intubation.
Conflicts of interestNone of the authors had conflicts of interest at the time the study was conducted. In the past 5 years, Dr. Pons has given talks for ResMed and Fisher & Paykel and collaborated in the development of a nasal interface for at-home use for Air Liquide Healthcare. The Hospital Sant Joan de Déu has equipment that has been donated by Philips and ResMed.
Please cite this article as: Pons-Òdena M, Medina A, Modesto V, Martín-Mateos MA, Tan W, Escuredo L, et al. ¿Cuáles son los factores predictores de fracaso de ventilación no invasiva más fiables en una unidad de cuidados intensivos pediátricos?. An Pediatr (Barc). 2019;91:307–316.
Previous presentations: Presentation of “Análisis de 10 años de experiencia en VNI en UCIP” at the XXIV Annual Congress of the Sociedad Española de Cuidados Intensivos Pediátricos held in Valencia, Spain, in 2009. Defence of doctoral dissertation at the Universitat de Barcelona, Spain, in June 2013.