Outcomes in patients diagnosed of acute lymphoblastic leukemia with Philadelphia chromosome (Ph-ALL) remains unfavourable compared to other subtypes of acute lymphoblastic leukemia despite improvements in drug treatments as well as advances in hematopoietic stem cell transplantation (HSCT).
Patients and methodsThe role of allogeneic HSCT in Ph-ALL patients has been analysed through a multicentric study where data belonging to 70 patients diagnosed of this entity in different centers that received HSCT between years 1998 and 2014, were reported by the Grupo Español de Trasplante Hematopoyético (GETH).
ResultsThe performance of HSCT from year 2004, in first complete remission (CR) status with thymoglobulin (ATG) based conditioning had a favorable impact on overall survival (OS). HSTC performance from year 2004, in first CR with ATG-based conditioning in addition to acute graft versus host disease (aGvHD) development, increased event free survival (EFS). Treatment with imatinib as well as undetectable minimal residual disease (MRD) prior to HSCT, combined with aGvHD, reduced risk of relapse (RR). Patient age less than 10 years when HSCT, first CR and ATG-based conditioning were associated to a lower transplant related mortality (TRM).
ConclusionsPatients that could achieve first CR that also received ATG-based conditioning had a better OS and EFS, so HSCT should be considered for this group of patients.
Los resultados de los pacientes con diagnóstico de leukemia linfoblástica aguda con cromosoma de Philadelphia (LLA-Ph) continúan siendo desfavorables comparados con los otros tipos de leucemias linfoblásticas agudas, pese a las mejoras en los tratamientos farmacológicos y los avances del trasplante de progenitores hematopoyéticos (TPH).
Pacientes y MétodosSe ha analizado el papel del TPH alogénico en pacientes diagnosticados de LLA-Ph mediante un estudio multicéntrico donde se recogen datos pertenecientes a 70 pacientes reportados por el Grupo Español de Trasplante Hematopoyético (GETH ), diagnosticados de esta enfermedad trasplantados en distintos hospitales españoles entre los años 1998 y 2014.
ResultadosLa realización del TPH a partir del año 2004, en primera remisión completa (RC) y con el empleo de timoglobulina (ATG) como parte del acondicionamiento, impactó favorablemente en la supervivencia global (SG). El TPH a partir del año 2004 en primera RC, así como el tratamiento con ATG y el desarrollo de enfermedad de injerto contra receptor aguda (EICRa), aumentaron la supervivencia libre de eventos (SLE). La administración de imatinib así como la ausencia de enfermedad mínima residual (EMR) previas al TPH, junto con la EICRa redujeron la probabilidad de recaída (PR). La edad del paciente inferior a 10 años, el estado de primera RC y el empleo de ATG en el acondicionamiento, disminuyeron la mortalidad relacionada con el TPH (MRT).
ConclusionesLos pacientes en primera RC que han recibido ATG durante el acondicionamiento presentan mayores SG y SLE. La indicación de TPH debería considerarse en estas situaciones.
Childhood cancer is the leading cause of death due to disease from the first year of life through adolescence. Acute lymphoblastic leukaemia (ALL) is the most frequent type of cancer in children.1 The mortality of ALL has decreased substantially in recent year, with 5-year overall survival rates increasing to up to 90%.2 However, the presence of genetic changes such as the t (9;22) translocation, found in 2–4% of paediatric patients with ALL, is associated with an increased risk of refractory disease and relapse.3
The chromosome that results from the aforementioned translocation, t (9;22) (q34;q11), is known as the Philadelphia chromosome. Nowell and Hungerford first described it in 1960 in adult patients with chronic myeloid leukaemia,4 and it has since been associated with the pathogenesis of some forms of acute myeloid leukaemia, ALL and mixed-phenotype acute leukaemia. The t (9;22) translocation of the tyrosine-protein kinase encoding gene ABL1 (chromosome 9) to the breakpoint cluster region BCR gene (chromosome 22) results in the fusion gene BCR-ABL1, which encodes a protein with constitutive tyrosine kinase activity.5 This results in permanent activation of cell proliferation pathways in haematopoietic stem cells, promoting pathogenicity through the inhibition of differentiation pathways and resistance to conventional treatment.6
Traditionally, a diagnosis of Philadelphia chromosome-positive ALL (Ph+ ALL) has been considered high-risk and to have a poor prognosis. Allogeneic haematopoietic stem cell transplantation (HSCT) has substantially improved survival in this disease compared to the exclusive use of chemotherapy.7,8
The addition of tyrosine kinase inhibitors (TKIs) to conventional chemotherapy has significantly improved the prognosis of Ph+ ALL, with the 5-year event-free survival increasing to approximately 70%,9,10 although still remaining lower compared to paediatric patients with Philadelphia chromosome-negative ALL.11
Imatinib is a TKI that acts by interacting with ABL, inhibiting its phosphorylation and therefore the activation of intracellular signal transduction cascades involved in proliferation. It also promotes apoptosis.12–14 Its combination with intensive chemotherapy followed by HSCT has been found to increase survival.15–18 However, it has not proven effective as monotherapy due to the development of drug resistance.11
The early response to treatment, determined by the measurement of minimal residual disease (MRD) through immunophenotyping or molecular techniques such as quantitative polymerase chain reaction (qPCR), is a very important prognostic factor in ALL, with more favourable outcomes in patients that have negative results for MRD.10,19–21
Patients treated with conventional chemotherapy and imatinib that achieve complete remission (CR) with negative MRD are considered low-risk. In this subset of patients, survival outcomes are not inferior to patients that also undergo HSCT. However, in the case of patients with positive MRD results after induction therapy, who are considered high-risk, survival can improve with performance of HSCT.9,15,22
At present, paediatric patients with a diagnosis of Ph+ ALL receive chemotherapy according to the protocol of the Sociedad Española de Hemato-Oncología Pediátrica (Spanish Society of Paediatric Haematology and Oncology, SEHOP) and the Spanish Haematology Treatment Programme (Spanish acronym, PETHEMA) 2013 update for the group of high-risk patients, by which imatinib is added from day 15 of conventional induction chemotherapy.
The primary objective of our study was to assess the clinical impact of HSCT in our region in paediatric patients with a diagnosis of Ph+ ALL by analysing the historical case series of the Spanish Group of Bone Marrow Transplantation in Children (Spanish acronym, GETMON) and the Spanish Group of Haematopoietic Stem Cell Transplantation (Spanish acronym, GETH).
Sample and methodsPatientsWe conducted a retrospective multicentre observational study in the 1998–2014 period that included 70 patients with a diagnosis of Ph+ ALL that underwent HSCT in 10 hospitals in Spain. The median age at diagnosis was 7.66 years (interquartile range [IQR], 6.67). Table 1 presents the clinical characteristics of the patients and the disease at the time of diagnosis.
Characteristics of the patients at the time of diagnosis of Philadelphia-positive ALL (n = 70).
| Participating hospitals | 10 |
|---|---|
| Median age in years at diagnosis [IQR] | 7.66 (6.67) |
| Sex, n (%) | |
| Male | 41 (58.57%) |
| Female | 29 (41.43%) |
| WBC count, cells × 109/L at diagnosis [IQR] | 45 [93.9] |
| CNS infiltration, n (%) | |
| Yes | 1 (1.43%) |
| No | 69 (98.57%) |
| MRD (BCR/ABL1 qPCR) after induction, n (%) | |
| Positive | 37 (52.86%) |
| Negative | 33 (47.14%) |
| Chemotherapy + imatinib n (%) | |
| Yes | 42 (60%) |
| No | 28 (40%) |
CNS, central nervous system; IQR, interquartile range; MRD, minimal residual disease; qPCR, quantitative polymerase chain reaction; WBC, white blood cell.
Patients were treated based on the SEHOP, PETHEMA and Berlin-Frankfurt-Münster (BFM) protocols.
Conducting the study did not involve modifying any of the information recorded in the existing databases or any form of direct contact with patients. We anonymised the data by assigning each patient a case number and limiting the collection of demographic data to those strictly necessary for the analysis of outcomes. The study was approved by the Clinical Research Ethics Committee of the Hospital Universitario La Paz.
Disease status at the time of HSCTWe included patients in first, second or subsequent complete remission. Minimal residual disease was measured by immunophenotyping or qPCR of the BCR/ABL1 gene. A total of 46 patients (65.72%) received imatinib: 30 patients exclusively before HSCT, 4 exclusively after HSCT and 12 both before and after HSCT. Some patients received other TKIs in addition to imatinib (Table 2).
Characteristics of haematopoietic stem cell transplantation.
| Median age in years at HSCT [IQR] | 9 (7.51) |
|---|---|
| Status before HSCT, n (%) | |
| 1st complete remission | 52 (74.28%) |
| 2nd complete remission | 9 (12.86%) |
| >2nd complete remission | 9 (12.86%) |
| BCR/ABL1 qPCR before HSCT, n (%) | |
| Positive | 30 (42.86%) |
| Negative | 36 (51.43%) |
| Imatinib, n (%) | |
| Pre-HSCT | 30 (42.86%) |
| Post-HSCT | 4 (5.71%) |
| Pre- and post-HSCT | 12 (17.14%) |
| Never | 24 (34.29%) |
| Other TKIs | |
| Dasatinib | 4 (5.7%) |
| Dasatinib and nilotinib | 3 (4.3%) |
| Type of donor, n (%) | |
| HLA-identical related donor | 24 (34.29%) |
| HLA-identical unrelated donor | 46 (65.71%) |
| Source of HSCs, n (%) | |
| UCB | 21 (30%) |
| BM | 32 (45.71%) |
| PB | 17 (24.29%) |
| Conditioning, n (%) | |
| Myeloablative | 55 (78.57%) |
| Reduced intensity | 15 (21.43%) |
| TBI n (%) | |
| Yes | 41 (58.57%) |
| No | 29 (41.43%) |
| GvHD prophylaxis with ATG, n (%) | |
| Yes | 41 (58.57%) |
| No | 29 (41.3%) |
| Median infused total nucleated cell dose, cells × 109/L [IQR] | 26.2 (45.77) |
| Graft failure, n (%) | 2 (2.86%) |
| Pharmacological immunosuppression, n (%) | |
| CsA | 10 (14.3%) |
| CsA + MTX | 38 (54.3%) |
| CsA + PRED | 16 (22.9%) |
| CsA + MPM | 3 (4.3%) |
| Acute GvHD, n (%) | |
| Grade II | 11 (15.71%) |
| Grade II–IV | 27 (38.57%) |
| Chronic GvHD, n (%) | 17 (24.29%) |
| Infection, n (%) | 24 (34.3%) |
| Relapse, n (%) | 15 (21.43%) |
| Status, n (%) | |
| Alive | 38 (54.28%) |
| Deceased | 32 (45.72%) |
| Cause of death, n (%) | |
| Relapse | 6 (18.75%) |
| Transplant-related | 26 (81.25%) |
| Cause of transplant-related death, n (%) | |
| Infection | 11 (42.31%) |
| GvHD | 7 (26.92%) |
| Idiopathic pneumonia | 4 (15.38%) |
| Haemorrhage | 1 (3.85%) |
| SOS | 1 (3.85%) |
| TMA | 1 (3.85%) |
| Second primary cancer | 1 (3.85%) |
| Median duration of follow-up, years (IQR) | 6.92 (12.25) |
ATG, thymoglobulin; BM, bone marrow; CsA, ciclosporin A; GvHD, graft versus host disease; HSC, haematopoietic stem cell; HSCT, haematopoietic stem cell transplantation; IQR, interquartile range; MPM, mycophenolate mofetil; MTX, methotrexate; PB, peripheral blood; PRED, prednisone; qPCR, quantitative polymerase chain reaction; TBI: total body irradiation; TKI, tyrosine kinase inhibitor; TMA, post-transplant thrombotic microangiopathy; TRM, transplant-related mortality; SOS, sinusoidal obstruction syndrome; UCB, umbilical cord blood.
In every case, the donor was an HLA-identical unrelated or related donor. The possible sources of haematopoietic stem cells were umbilical cord blood, bone marrow or peripheral blood. We defined myeloablative conditioning as a conditioning regimen that included total body irradiation (TBI) or administration of high-dose busulfan, and reduced intensity conditioning as a regimen combining fludarabine and alkylating agents. The post-transplant immunosuppression treatment and its duration were based on the criteria established in each facility. We included treatment with anti-thymocyte globulin (ATG) for prophylaxis against graft-versus-host disease (GvHD) as a study variable. Table 2 presents the rest of the variables associated with HSCT.
Post-transplant complicationsWe counted all cases of graft failure (both primary and secondary) as a single outcome variable. We defined GvHD according to the criteria established by the National Institutes of Health (NIH).23,24 For the purpose of the study, we categorised acute GvHD as mild (grade I) and moderate-severe (grades II–IV). We collected data on the infections that had been considered severe in each centre due to requiring hospital admission or another form of intervention.
Relapse, status at the end of follow-up and transplant-related mortalityWe recorded the frequency of patients that had any type of relapse (medullary, extramedullary or combined) during the follow-up and the status of the patient (alive or deceased) at the end of recruitment. The follow-up of patients lasted until their death or otherwise November 2020.
We defined transplant-related mortality (TRM) as death due to causes other than leukaemia relapse. Table 2 summarises the causes of death in TRM.
Statistical analysisThe statistical analysis was conducted with the software Statistical Package for the Social Sciences (SPSS) version 26.0 (SPSS Inc; Chicago, IL, USA). We have expressed qualitative variables as absolute and relative frequencies. In the case of quantitative variables, we used the median as a measure of central tendency and the IQR as the measure of dispersion. We compared qualitative variables with the chi square test. In the survival analysis, we obtained the overall survival (OS), event-free survival (EFS) the probability of relapse (PR) and the TRM using the Kaplan-Meier method. We compared these results with the log-rank test, expressing it as the percentage with its 95% confidence interval (CI) or standard deviation (SD). We analysed the association of the variables under study with the OS, EFS, PR and TRM. First, we conducted a univariate analysis of the different variables using the Cox proportional hazards model to obtain the corresponding hazard ratios. Subsequently, variables with a significant association (P < .1) were included in a multivariate Cox regression analysis with stepwise variable selection. We defined statistical significance as a P value of 0.05 or less.
ResultsFifty-two patients (74.28%) underwent HSCT in first CR, 9 (12.86%) in second CR and 9 (12.86%) in subsequent remissions. In 36 patients (51.4%), the results of MRD were negative before HSCT.
The source of haematopoietic stem cells was bone marrow in 32 patients (45.7%), in 13 cases (18.57%) from HLA-identical related donors, umbilical cord blood in 21 patients (30%), in 1 case from an HLA-identical related donor, and mobilised peripheral blood in 17 patients (24.3%), in 10 cases (14.28%) from HLA-identical related donors.
The conditioning regimen was myeloablative in 55 of the HSCTs (78.57%) and included TBI in 41 (58.57%). Conditioning included administration of ATG in 41 patients (58.57%). All patients received cyclosporin, alone or combined with another agent, for post-transplant immunosuppression (Table 2).
Two (2.86%) patients experienced graft failure. The most frequent post-transplant complication was GvHD, in the form of acute GvHD in 38 patients (54.3%) and chronic GvHD in 17 (24.3%).
Of the patients that developed acute GvHD, 11 (28.9%) had mild presentations, while 27 (71.1%) developed moderate to severe disease. Infectious complications developed in 24 patients (34.3%) and were the second most frequent type of complication.
A total of 15 patients (21.43%) experienced relapse. There were 32 deaths (45.72%): 6 (18.75%) due to relapse and 26 (81.25%) transplant-related (Table 2).
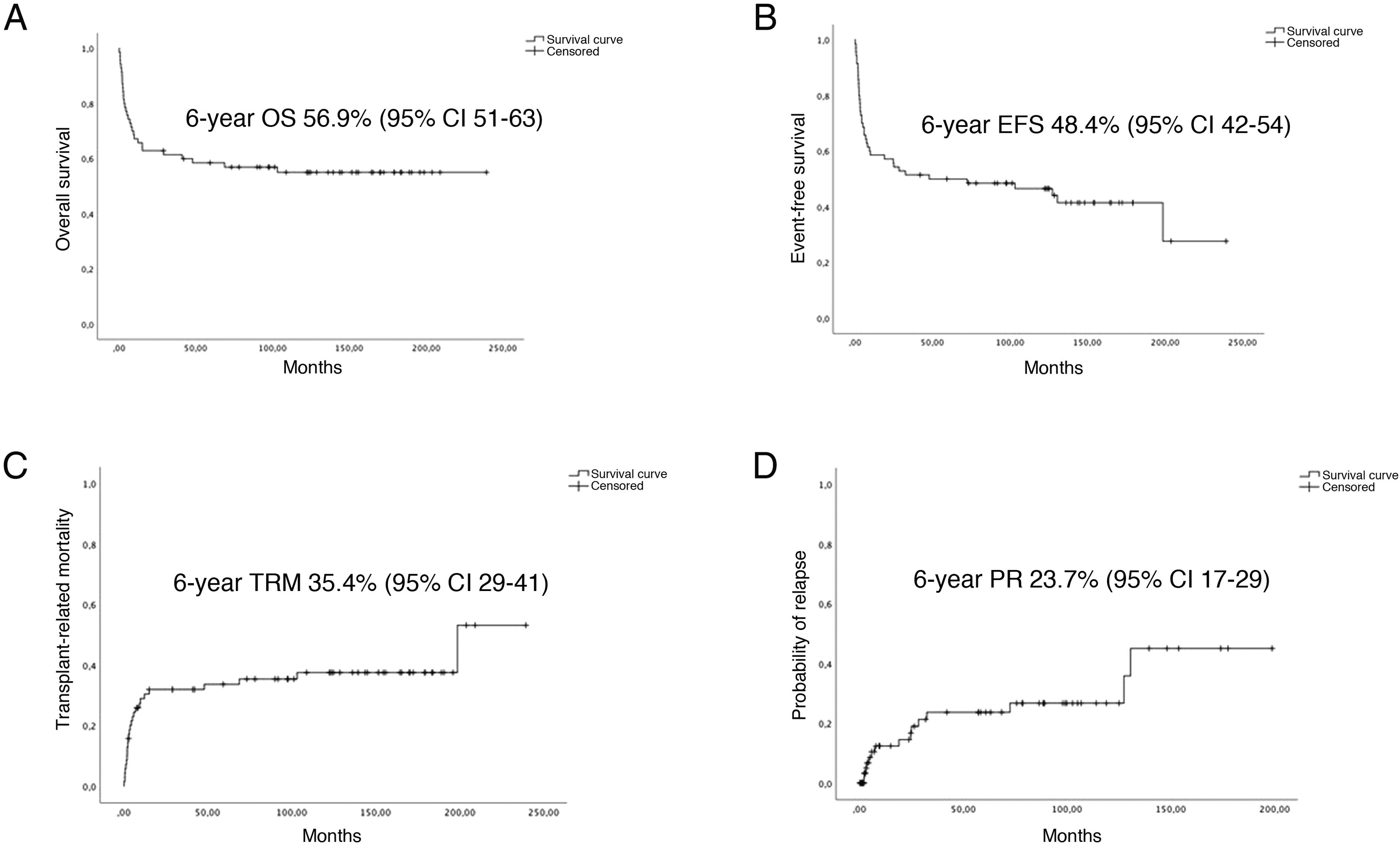
The median duration of follow-up in the cohort was 6.92 years (IQR, 12.25). The 6-year OS was 56.9% (SD, 5.9%) and the 6-year EFS 48.4% (SD, 6%). The 6-year PR was 23.7% (SD, 6.1%) and the 6-year TRM was 35.7% (SD, 6%) (Fig. 1).
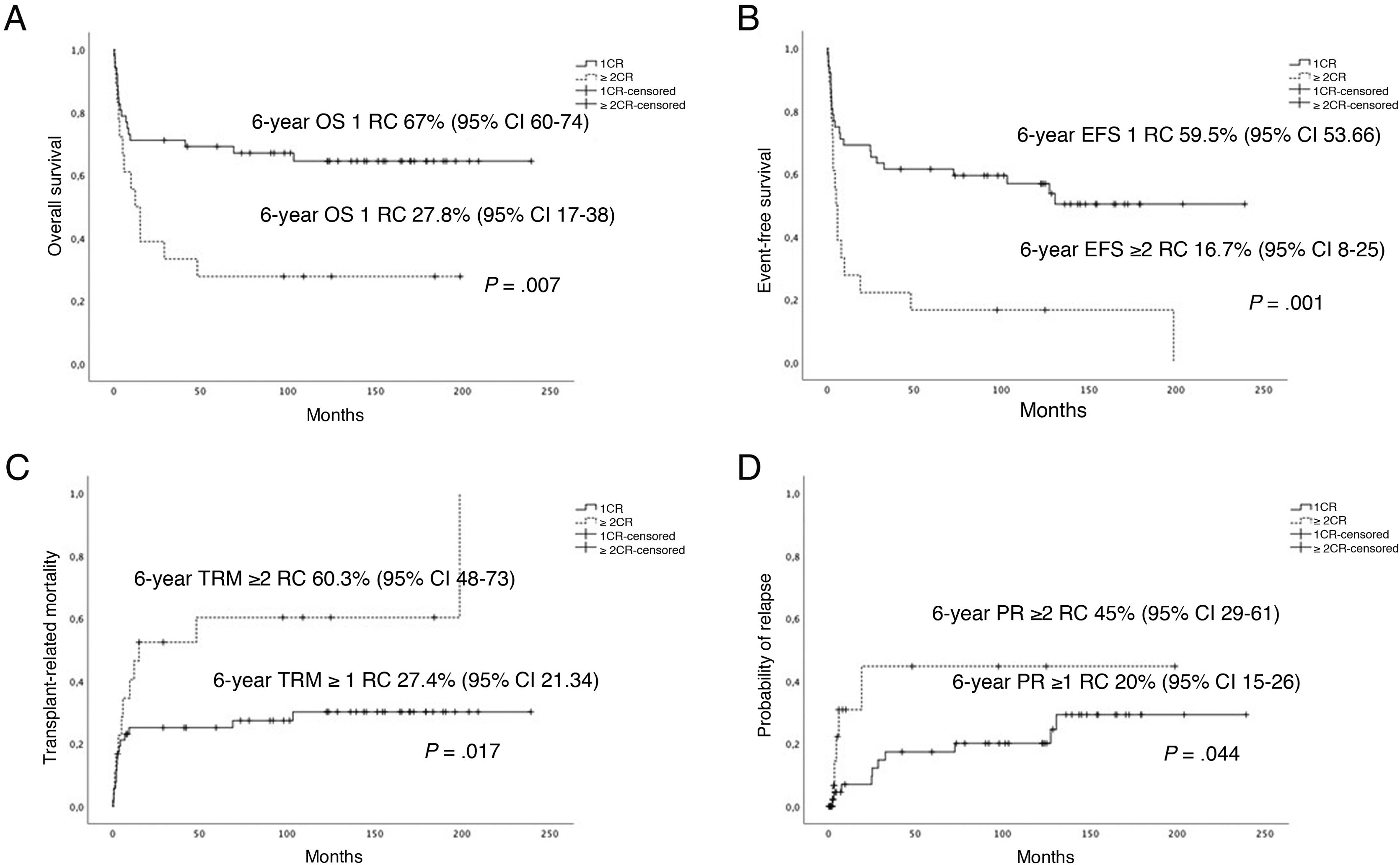
Fig. 2 presents the statistically significant differences in OS, EFS, PR and TRM in patients that underwent transplantation in first CR.
The use of imatinib increased significantly from 2004. In addition, the lowest proportion of relapse occurred in the group of patients treated with imatinib exclusively before HSCT (10%; P = .006), compared to patients who did not receive imatinib, who received it both before and after HSCT or who received it only after HSCT (Table 3).
Frequency distribution based on timing of imatinib.
| Variable | No imatinib | Imatinib-pre | Imatinib-post | Imatinib-pre + post | Total | P |
|---|---|---|---|---|---|---|
| (N = 24) | (N = 30) | (N = 4) | (N = 12) | (N = 70) | ||
| Year of HSCT | ||||||
| <2004 | 17 (70.8%) | 0 (0%) | 2 (50%) | 0 (0%) | 19 (27.1%) | .000 |
| >2004 | 7 (29.2%) | 30 (100%) | 2 (50%) | 12 (100%) | 51 (72.9%) | |
| Relapse | 4 (16.7%) | 3 (10%) | 3 (75%) | 5 (41.7%) | 15 (21.4%) | .006 |
| Death | 15 (62.5%) | 10 (33.3%) | 3 (75%) | 4 (33.3%) | 32 (45.71%) | .08 |
CR, complete remission; HSCT, haematopoietic stem cell transplantation; MRD, minimal residual disease.
Performance of HSCT in 2004 or after, first CR status and administration of ATG during conditioning were positively associated with OS. Performance of HSCT in 2004 or after, first CR status, administration of ATG during conditioning and development of acute GvHD were associated with a better EFS. When it came to the PR, treatment with imatinib, negative MRD and the development of acute GvHD had a protective effect. In the analysis of TRM, age less than 10 years, first CR status and use of ATG as part of the conditioning regimen had a favourable impact (Table 4).
Univariate analysis of overall survival, event-free survival, probability of relapse and transplant-related mortality.
| 6-year OS | 6-year EFS | 6-year PR | 6-year TRM | |||||
|---|---|---|---|---|---|---|---|---|
| % (SD) | P | % (SD) | P | % (SD) | P | % (SD) | P | |
| General | 56.9 (5.9) | 48.4 (6) | 23.7 (6.1) | 35.4 (6) | ||||
| Year of HSCT | ||||||||
| <2004 | 36.8 (11) | .025 | 31.6 (10.7) | .047 | 28.7 (14.1) | .669 | 47.4 (11) | .09 |
| ≥2004 | 64.4 (6.8) | 54.8 (7) | 22.2 (6.7) | 30.7 (6) | ||||
| Age | ||||||||
| <10 years | 62.9 (7.1) | .163 | 52 (7.4) | .452 | 22.5 (7.1) | .903 | 31.4 (7) | .05 |
| ≥10 years | 45.5 (10.2) | 41.7 (10) | 26.8 (11.9) | 43 (10.3) | ||||
| Donor | ||||||||
| Related | 57.8 (10.2) | .598 | 54.2 (10) | .3 | 25.1 (9.8) | .742 | 26.9 (9.6) | .27 |
| Unrelated | 56.3 (7.3) | 41.7 (10) | 22.4 (7.6) | 37.4 (7.2) | ||||
| Imatinib | ||||||||
| No | 41.7 (10.1) | 37.5 (9.9) | 83.9 (10.4) | 51 (10) | ||||
| Pre- HSCT | 69.2 (8.6) | .101 | 62.2 (8.8) | .07 | 13 (7.1) | .009 | 30.8 (8.6) | .13 |
| Post-HSCT | 25 (21.7) | 25 (21) | 37.5 (2.86) | 25 (21.7) | ||||
| Pre- and post-HSCT | 66.7 (13.6) | 41.7 (14.2) | 49.1 (16.4) | 17.5 (11) | ||||
| MRD | ||||||||
| Positive | 60.9 (8.2) | .365 | 39.7 (9) | .14 | 50 (35.4) | .009 | 37.8 (9) | .5 |
| Negative | 56.5 (9.1) | 58.3 (8.2) | 13.4 (6.3) | 31 (7.9) | ||||
| Pre-HSCT status | ||||||||
| 1st CR | 67.1 (6.6) | .02 | 59.5 (6.8) | .003 | 17.7 (6.1) | .134 | 27.4 (6.3) | .05 |
| 2nd CR | 11.1 (10.5) | 11.1 (0.5) | 41.7 (25.1) | 77.8 (17.8) | ||||
| >2nd CR | 44.4 (16.6) | 22.2 (13.9) | 50 (20.4) | 46.7 (17.3) | ||||
| ATG | ||||||||
| Yes | 70.7 (7.1) | .008 | 58.5 (7.7) | .025 | 23.7 (7.4) | .736 | 20.2 (7) | .004 |
| No | 37.2 (9.1) | 34.5 (8.8) | 22.1 (10.2) | 57.1 (9.6) | ||||
| TBI | ||||||||
| No | 52.9 (9.2) | .745 | 42.8 (9.1) | .295 | 30.9 (10.6) | .119 | 38.1 (9.2) | .7 |
| Yes | 59.9 (7.8) | 52.5 (7.9) | 18.2 (6.8) | 33.6 (8.1) | ||||
| Acute GvHD | ||||||||
| No | 54.7 (6.8) | .431 | 37.5 (8.6) | .007 | 39.4 (10.3) | .009 | 36 (8.7) | .8 |
| Yes | 63.3 (12) | 57 (8) | 11.3 (6.3) | 35.2 (7.9) | ||||
| Chronic GvHD | ||||||||
| No | 50 (8) | .365 | 45.1 (6.9) | .237 | 24.8 (7.3) | .335 | 36.5 (6.7) | .781 |
| Yes | 62.9 (7.9) | 58.8 (11.4) | 21.6 (11.2) | 31.4 (11.7) | ||||
ATG, thymoglobulin; CR, complete remission; EFS, event-free survival; GvHD, graft-versus-host disease; HSCT, haematopoietic stem cell transplantation; MRD, minimal residual disease; OS, overall survival; PR, probability of relapse; TRM, transplant-related mortality.
Administration of ATG during conditioning, performance of HSCT in the first CR and performance of HSCT in or after 2004 were positively associated with OS. Performance of HSCT in the first CR, use of ATG and performance of HSCT in or after 2004 were associated with an increased EFS. Administration of imatinib combined with undetectable MRD before HSCT was associated with a decreased PR. Lastly, use of ATG during conditioning and performance of HSCT from year 2004 were associated with a lower TRM (Table 5).
Multivariate analysis of overall survival, event-free survival, probability of relapse and transplant-related mortality.
| Outcome | Parameter | P | HR | 95% CI |
|---|---|---|---|---|
| OS | ATG | .001 | 0.232 | 0.095−0.567 |
| Pre-HSCT status | .025 | 1.722 | 1.069−2.78 | |
| HSCT from 2004 | .009 | 3.288 | 1.343−8.05 | |
| EFS | Pre-HSCT status | .002 | 1.832 | 1.251−2.682 |
| ATG | .007 | 0.387 | 0.194−0.771 | |
| HSCT from 2004 | .01 | 2.537 | 1.235−5.13 | |
| PR | Imatinib | .021 | 2.585 | 1.155−5.788 |
| MRD | .023 | 10.5 | 1.388−79.447 | |
| TRM | ATG | .002 | 0.249 | 0.106−0.589 |
| HSCT from 2004 | .17 | 2.82 | 1.199−6.631 |
ATG, thymoglobulin; CI, confidence interval; EFS, event-free survival; HR, hazard ration; HSCT, haematopoietic stem cell transplantation; MRD, minimal residual disease; OS, overall survival; PR, probability of relapse; TRM, transplant-related mortality.
Our study summarises the experience in Spain with HSCT in paediatric patients with Ph+ ALL, an infrequent disease with a poor prognosis, over a long 22-year period.
The data evinces that the use of imatinib before HSCT has a relevant role, with a substantial impact in the probability of relapse. This was consistent with the findings of previous studies.9,25,26 However, it is important to note that some authors question the impact of imatinib in terms of increasing survival.27,28
Overall, the data showed progressive improvement in HSCT outcomes. This was a significant trend evinced in increases in OS and EFS and decreases in TRM performed from 2004. This inflection point may be explained by the increased cumulative experience, the optimization of donor selection, conditioning strategies, advances in immunosuppressive therapy and adequate prophylaxis.29
Another significant factor was disease status before HSCT. In patients that underwent HSCT when they were in first CR, the OS and EFS were higher and the TRM lower. On the other hand, the absence of detectable MRD was associated with a lower PR. In the univariate analysis, the age of the patient at the time of transplantation appeared to be an important factor, as TRM was lowest in patients aged less than 10 years.26
The increased survival observed in patients treated with chemotherapy and TKI suggests that not all patients with Ph+ ALL may require treatment with HSCT after achieving first CR. The COG-AALL0031 study found that patients treated with chemotherapy combined with imatinib who had not undergone HSCT had a 5-year EFS of 70% (SD, 12%), without significant differences compared to patients that received a sibling donor bone marrow transplant (65% ± 11%) or an unrelated donor transplant (59% ± 15%).10 In the EsPhALL2010 study, treatment consisted of imatinib and chemotherapy. Haematopoietic stem cell transplantation was performed in high-risk patients and in low-risk patients with access to an HLA-identical donor. The 5-year EFS was 62.7% in low-risk patients (95% CI, 52–71.6) and 46.3% in high-risk patients (95% CI, 32.3–59.2). There was a significant increase in EFS in low-risk patients treated with chemotherapy and imatinib compared to patients that also underwent HSCT during the first CR.9,26
Thus, the option of HSCT should be contemplated in patients that do not achieve an undetectable MRD at the end of induction, and not routinely, due to the favourable outcomes achieved with chemotherapy combined with TKIs in low-risk patients. In our study, the use of imatinib was associated with significant improvements in HSCT outcomes from year 2004, with a decreased frequency of relapse. However, there are contradictory results in the literature as regards patients treated with TKIs.25,26,30
In the cohort under study, there was a significant number of patients who developed GvHD, both in its acute and chronic forms, but it did not ultimately have a negative impact on survival. This has been observed in the past.27 Moreover, we ought to highlight that patients who developed acute GvHD had a higher EFS and lower PR in the univariate analysis. This could be explained by the graft-versus-leukaemia effect associated with GvHD. Other studies have associated the development of chronic GvHD with a lower relapse rate, suggesting that it may be a protective factor.31
Treatment with ATG as part of the conditioning regimen had a positive impact on OS and EFS. It was associated with a decreased TRM, so it acts as a protector against the development of severe forms of GvHD, as described in other studies.32–34
The use of TBI in children is currently questioned due to its important late side effects.35 A recent study36 did not find a significant association of TBI with survival or PR in children that underwent TBI prior to HSCT, and instead found an association with the development of GvHD. The findings of our retrospective study were similar.
As we already mentioned, in our study we found the strongest association of undetectable MRD with a decrease in PR. However, the data available for study did not allow us to determine which of the techniques used to quantify MRD (flow cytometry vs molecular methods) is more appropriate for the purpose.36
Compared to previous studies, we did not find differences between patients that received the transplant from an HLA-identical related donor versus an HLA-identical unrelated donor.8,37 This is quite relevant, as an HLA-identical related donor will not be available in most cases.
In light of the above, we think it is relevant to share our findings, especially given the current importance of CAR T-cell therapies in B-cell ALL. However, it is important to keep in mind that the indication of CAR T-cell therapy in the case of Ph+ ALL is restricted to patients who cannot tolerate or do not respond to 2 TKIs or in whom the use of TKIs is contraindicated. In spite of these, favourable outcomes have been reported with the use of CAR T cells.38–40
There were limitations to this study, chiefly related to its retrospective design and the prolonged period under study.
ConclusionImatinib has a beneficial impact on disease status before transplantation, promoting complete remission and MRD negativity and therefore decreasing the probability of relapse.
In patients in first complete remission that received ATG, the OS and EFS were higher, so the use HSCT should be considered in these patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the European Regional Development Fund (ERDF) and Instituto de Salud Carlos III health research grant (FIS PI18/01301) and the Fundación Cris contra el Cáncer (http://criscancer.org) for their support.
Please cite this article as: Galán Gómez V, Fuente Regaño L, Rodríguez Villa A, Heredia Rubio CD, González Vicent M, Badell Serra I, et al., Experiencia del Grupo Español de Trasplante Hematopoyético (GETMON-GETH) en el trasplante alogénico de progenitores hematopoyéticos en leucemia aguda linfoblástica Philadelphia, Anales de Pediatría. 2022;96:309–318.