Asparaginase (ASP) is an essential cytostatic agent in the treatment of paediatric acute lymphoblastic leukaemia, with evidence of an improvement in event-free survival since it was first introduced.1,2 This enzyme hydrolyses asparagine, an amino acid that the lymphoblasts require from exogenous sources and whose depletion therefore induces their apoptosis.1 The 3 most widely used formulations are those derived from Escherichia coli, either in its native form (native-ASP) or pegylated (PEG-ASP) and the one derived from Erwinia chrysanthemi (Erwinia-ASP).2
Despite the benefits of ASP, between 20% and 25% of patients develop serious adverse events.1 Hypersensitivity reactions are among the most frequent ones (13%–22%) and are caused by the formation of antibodies that neutralise the enzyme, reducing asparaginase activity (AA) and its efficacy.1–3 There are 2 types of hypersensitivity reactions, reactions manifesting with clinical allergy (13%–15%) and asymptomatic reactions resulting in silent inactivation (3%–8%).3 In both cases, the use of ASP derived from E coli should be discontinued, switching to Erwinia-ASP.
Since 2016, the updated, second version of the LAL/SEHOP-PETHEMA acute lymphoblastic leukaemia treatment protocol originally published in 2013 by the Sociedad Española de Hematología y Oncología Pediátrica (SEHOP, Spanish Society of Paediatric Haematology and Oncology) recommend use of PEG-ASP from induction in every arm: 3 doses in the standard risk arm, 13 in the intermediate risk (IR) arm, and 11 in the high-risk (HR) arm.4
The assessment of AA, therefore, allows identification of silent inactivation and the differentiation of real allergic reactions from infusion-related reactions (pseudoallergy) with a similar presentation but in which AA levels continue to be good, thereby allowing continued treatment with PEG-ASP with premedication of the patient and drug monitoring.1
In this article, we present our experience with therapeutic AA monitoring in paediatric patients with acute lymphoblastic leukaemia treated with this protocol in our centre and the analysis of cases of PEG-ASP hypersensitivity.
We conducted a single centre retrospective observational study between June 2016 and January 2022 in a tertiary care hospital. Asparaginase activity was measured 7 or 14 days (±1 day) after administration of PEG-ASP and at 48 h in the case of Erwinia-ASP. Enzymatic activity was measured in samples of EDTA-anticoagulated blood with a quantitative assay that used l-aspartic acid β-hydroxamate as the substrate and measured the indooxine formation by photometry at 690 nm.5
We defined silent inactivation as an AA after administration of PEG-ASP of less than 100 IU/L on day 7 ± 1; less than 20 IU/L on day 14 ± 1 or an AA after administration of Erwinia-ASP of less than 20 IU/L at 48 h post administration.2 The results were confirmed with a second measurement in every instance.6 We present the results expressed in terms of percentages and median and interquartile range (IQR).
The sample included 24 patients (12 male), with a median age of 6.7 years (IQR, 4–12.7), 4.2% (n = 1) in the standard risk group; 66.7% (n = 16) in the IR group and 29.2% (n = 7) in the HR group. Fifty percent of patients had completed treatment at the time of the study.
The analysis included a total of 125 measurements of AA. The median level was 401 IU/L (IQR, 225–562.3). The percentage of AA measurements per patient performed out of the total that could have been performed ranged between 30% and 100%, with a median percentage of 62% (IQR, 54–77).
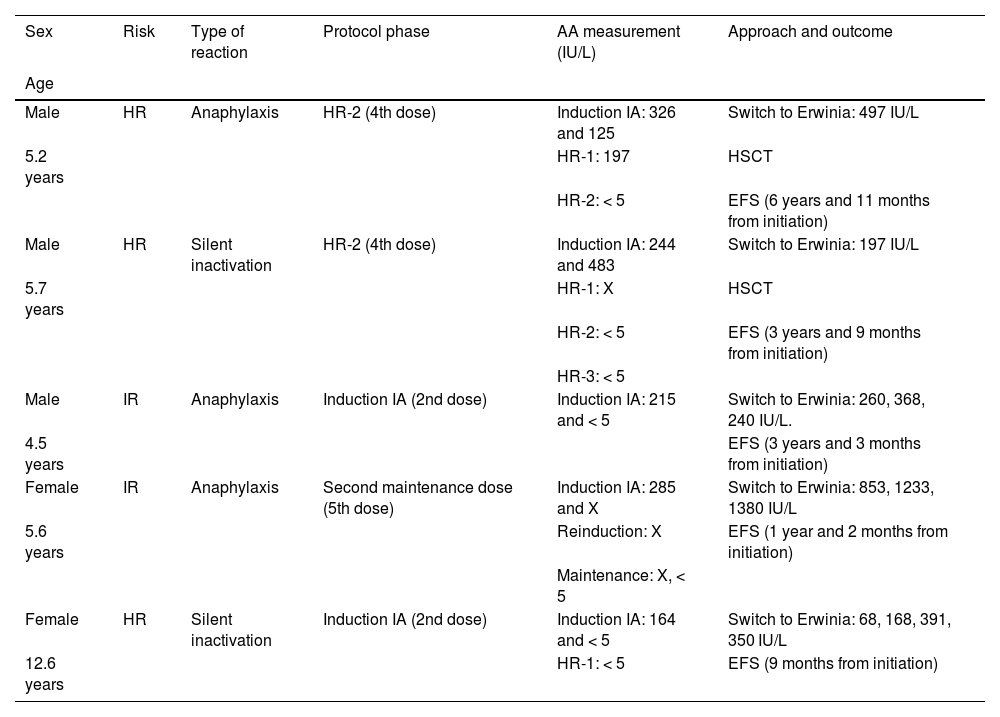
A total of 5 patients had hypersensitivity reactions: 3 (12.5%) with an anaphylactic reaction (with confirmation of levels of AA < 5 IU/L after the event) and 2 patients (8.3%), both in the HR group, in the form of silent inactivation. In all 5 (20.8% of the sample), treatment was switched to Erwinia-ASP, which was well tolerated and with no subsequent evidence of inactivation (Table 1). None of the patients had pseudoallergy. Two patients in the HR group had undetectable levels of AA after reinduction, however, they had sustained normal levels after the maintenance doses, which ruled out silent inactivation.
Patients with hypersensitivity reactions to asparaginase, phase of protocol in which it was detected and approach to management.
| Sex | Risk | Type of reaction | Protocol phase | AA measurement (IU/L) | Approach and outcome |
|---|---|---|---|---|---|
| Age | |||||
| Male | HR | Anaphylaxis | HR-2 (4th dose) | Induction IA: 326 and 125 | Switch to Erwinia: 497 IU/L |
| 5.2 years | HR-1: 197 | HSCT | |||
| HR-2: < 5 | EFS (6 years and 11 months from initiation) | ||||
| Male | HR | Silent inactivation | HR-2 (4th dose) | Induction IA: 244 and 483 | Switch to Erwinia: 197 IU/L |
| 5.7 years | HR-1: X | HSCT | |||
| HR-2: < 5 | EFS (3 years and 9 months from initiation) | ||||
| HR-3: < 5 | |||||
| Male | IR | Anaphylaxis | Induction IA (2nd dose) | Induction IA: 215 and < 5 | Switch to Erwinia: 260, 368, 240 IU/L. |
| 4.5 years | EFS (3 years and 3 months from initiation) | ||||
| Female | IR | Anaphylaxis | Second maintenance dose (5th dose) | Induction IA: 285 and X | Switch to Erwinia: 853, 1233, 1380 IU/L |
| 5.6 years | Reinduction: X | EFS (1 year and 2 months from initiation) | |||
| Maintenance: X, < 5 | |||||
| Female | HR | Silent inactivation | Induction IA (2nd dose) | Induction IA: 164 and < 5 | Switch to Erwinia: 68, 168, 391, 350 IU/L |
| 12.6 years | HR-1: < 5 | EFS (9 months from initiation) |
AA, asparaginase activity; EFS, event-free survival (to date); HR, high-risk; HSCT, haematopoietic stem cell transplantation; IR, intermediate risk; X, undetermined.
Monitoring of AA is not currently included in the routine management protocol in most centres, yet it is recommended by experts6 and should follow administration of each dose of ASP independently of whether the patient exhibits signs and symptoms of hypersensitivity. Measurement of AA allowed the detection of 2 patients in the high risk group with silent inactivation and optimization of treatment by switching to Erwinia-ASP before they underwent haematopoietic stem cell transplantation. It also allowed confirmation of actual hypersensitivity in the 3 patients with anaphylactic reactions, avoiding unnecessary changes in treatment. Despite the small sample size, the observed percentages of allergy (12.5%) and inactivation (8.3%) were similar to those described in the previous literature.3 These findings support the importance of routine AA monitoring in the management of acute lymphoblastic leukaemia.
Previous meeting: this study was presented at the XIV National Congress of the Sociedad Española de Hematología y Oncología Pediátricas; May 26–28, 2022; Badajoz, Spain.