Fighting against antimicrobial resistance is a current priority, and further efforts need to be made to improve antimicrobial prescribing and reduce the spread of infections in paediatric care settings.
MethodsWe conducted a prospective longitudinal study on the use of antimicrobials from the time the antimicrobial stewardship programme (ASP) was introduced in January 2016 to December 2017 (period 2 [P2]) in our children’s hospital. We compared the obtained results on antimicrobial prescribing with retrospective data from the period preceding the introduction of the ASP (2014–2015, period 1 [P1]). The sample consisted of paediatric inpatients who received broad-spectrum antimicrobials, antifungals or intravenous antibiotherapy lasting more than 5 days. We compared the use of antimicrobials in P1 versus P2.
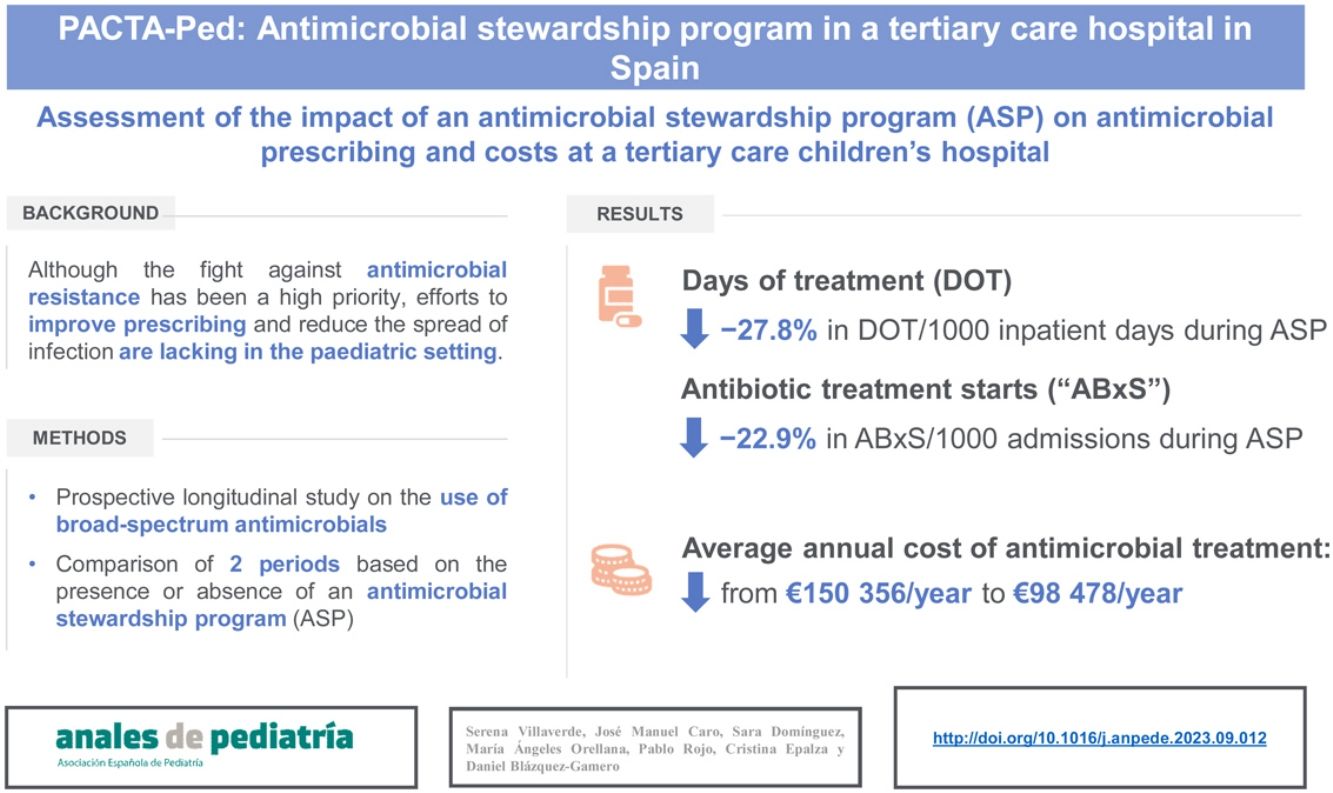
ResultsA total of 160 patients were included during P2. The antibiotics for which a recommendation was made most frequently were meropenem (41.6%) and cefotaxime (23.4%). In 45% of care episodes, the consultant recommended “no change” to the prescribed antimicrobial. The final rate of acceptance of received recommendations by the prescribing physicians was 89%. We found average decreases of 27.8% in the days of treatment per 1000 inpatient days and 22.9% in the number of antimicrobial starts per 1000 admissions in P2. The use of carbapenems, cephalosporins and glycopeptides decreased in P2 compared to P1. The average annual cost of antimicrobial treatment decreased from є150 356/year during P1 to є98 478/year in P2.
ConclusionOur ASP achieved a significant decrease in the use of broad-spectrum antibiotics and antifungals. The costs associated with antimicrobial prescribing decreased following the introduction of the ASP, which was a cost-effective action in this study period.
La lucha contra la resistencia a los antimicrobianos es actualmente prioritaria; son necesarios esfuerzos para mejorar la prescripción y reducir la propagación de infecciones en el entorno pediátrico.
MétodosEstudio longitudinal prospectivo sobre el uso de antimicrobianos realizado desde el inicio del programa de optimización del uso de antimicrobianos (PROA) en enero de 2016 hasta diciembre de 2017 (período 2; P2) en el hospital infantil. Los resultados obtenidos se han comparado retrospectivamente con el periodo anterior al inicio del PROA (2014–2015, periodo 1; P1). La población estudiada fueron niños ingresados que recibieron antimicrobianos de amplio espectro, antifúngicos o tratamiento antibiótico intravenoso más de 5 días.
ResultadosDurante el P2 se incluyeron un total de 160 pacientes. Los antibióticos más comunes para los que se realizó una recomendación fueron: meropenem (41,6%) y cefotaxima (23,4%). En el 45% de los episodios se recomendó “no cambiar” el antimicrobiano prescrito. La tasa de aceptación de las recomendaciones por parte de los facultativos responsables fue del 89%. Se objetivó una disminución promedio de 27,8% y 22,9% en los días de tratamiento (DOT)/1000 días de ingreso y el número de tratamientos iniciados/1000 ingresos, respectivamente. El uso de carbapenémicos, cefalosporinas y glucopéptidos disminuyó de P1 a P2. El coste medio anual del tratamiento antimicrobiano pasó de 150 356 euros/año durante el P1 a 98 478 euros/año en el P2.
ConclusionesNuestro PROA conllevó una disminución significativa en el uso de antibióticos y antifúngicos de amplio espectro. Los costes asociados con la prescripción de antimicrobianos disminuyeron desde el inicio del PROA y resultó una acción coste-efectiva durante el período de estudio.
The burden of antimicrobial resistance (AMR) has been highlighted by the most relevant health and social agencies, which have included AMR in the list of the ten major threats to global health in 2019 and warned that AMR could cause catastrophic damage to the global economy.1,2 Southern European countries have the highest rates of AMR in the European Union (EU).3 A recent and comprehensive systematic review has yielded data on the global burden of bacterial AMR in 2019.4 Its authors estimated that there were 4.95 million deaths associated with bacterial AMR in 2019.4 The paediatric population was severely affected, with deaths in children under 5 years amounting to 20% of the total.4 Moreover, the United Nations Children’s Fund (UNICEF) estimated that 40% of deaths globally among children under 5 years of age and neonates were caused by diseases whose treatment was directly affected by AMR in 2016.5 In the EU, the evidence shows that infants under 1 year presented the highest burden of attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in 2015.3 Infections caused by AMR bacteria represent a high burden in terms of deaths and disability-adjusted life-years in the paediatric population, not only at the global level but also in the EU.6 The irrational use of antibiotics in humans is one of the main causes of the increase in AMR, and it is estimated that up to two-thirds of antibiotic prescriptions made globally are inappropriate.7 Antibiotics are the most common prescribed drugs in the paediatric population.8,9 Children in Southern Europe are more likely to receive antibiotics than children in Northern Europe.10 Moreover, fewer than 70% of prescriptions for children made in the community in Italy and Greece are for narrow-spectrum antibiotics, compared, for example, with Slovenia, Netherlands or Spain, where these drugs account for more than 90% of cases.11 Similar prescribing patterns have also been identified in the hospital setting.12
The increase in AMR and the need to contain pharmaceutical costs have generated considerable interest in the development of programmes to optimize the use of antibiotics.13 Antimicrobial stewardship is defined as any activity that promotes the selection of the optimal dosing, route of administration, and duration of therapy for medications administered to treat infections.14 There is ample evidence of the benefits of antimicrobial stewardship programmes (ASPs) in adult care settings across the world.15,16
Although the fight against AMR has been considered a priority for some time, a recent systematic review found that very few ASPs for paediatric patients had been developed in Europe compared to the United States (US).9 However, since 2007, there has been a marked increase in the proportion of hospitals providing paediatric services with formal ASPs.17 In 2005, our hospital began an ASP called PACTA (non-compulsory antibiotic counselling and surveillance program) in inpatient adult wards.18 Since 2016, the PACTA paediatric programme (PACTA-Ped) has been implemented in the children’s hospital. The objective of our study was to evaluate the impact of the PACTA-Ped programme on antimicrobial prescribing.
MethodsWe conducted a prospective longitudinal study focused on the use of antimicrobials in the department of paediatrics of our tertiary care children’s hospital from the introduction of the PACTA-Ped program in January 2016 to December 2017 (period 2 [P2]). We compared the obtained results on antimicrobial prescribing to retrospective data from the period prior to the start of the ASP (2014–2015, period 1 [P1]).
Study populationThe sample consisted of children admitted to our hospital treated with broad-spectrum antimicrobials (defined below), antifungals or intravenous (IV) antibiotherapy for more than 5 days. The study was conducted in a 145-bed tertiary care children’s hospital. The centre has a 19-bed neonatal intensive care unit and a 16-bed paediatric intensive care unit, manages 3000 admissions per year and its services include complex general paediatric surgery, surgery for treatment of congenital heart defects and paediatric oncological care. We recorded data on demographic characteristics, the unit of admission, type and length of antibiotic/antifungal treatment, days of therapy (DOT), DOT per 1000 inpatient days (DOT/1000), antibiotic starts (ABxS) for each targeted antibiotic and antibiotic starts per 1000 hospital admissions (ABxS/1000) as aggregate data. The information was collected and stored with the REDCap® data collection system.19 The data were hosted in a secure online server located at the research institute of the hospital (Instituto de Investigación Hospital 12 de Octubre). The study was exempt from informed consent, as it did not pose any risks to participants and did not entail changes to patient care. The study was approved by the research ethics committee of the hospital (code: TP17/0330).
Study proceduresSince the beginning of the PACTA-Ped program, a paediatric infectious diseases (PID) consultant reviewed all antimicrobial prescriptions made at the children’s hospital through a software tool designed for the purpose twice a week (Tuesday and Thursday). Prescribing at the intensive care units (neonatal and paediatric) could not be evaluated because they did not have electronic prescription software at the time of the study. The antimicrobials considered to be “broad-spectrum” were amoxicillin-clavulanate, cefotaxime/ceftriaxone, ceftazidime, cefepime, ceftolozane-tazobactam, piperacillin-tazobactam, meropenem, imipenem, teicoplanin, vancomycin, linezolid, quinolones (IV and oral), colistin, tigecycline, and daptomycin. All antifungals were also included in this category. Other novel antibiotics (ceftazidime-xavibactam, cefiderocol) were not used during the study period, so they were not evaluated. Dosing adjustment by age, weight and renal function is performed in each prescription. Plasma antimicrobial monitoring is not performed routinely.
As a complement to this surveillance of antimicrobial prescribing, daily sessions were held with the Microbiology Department, where all positive results from blood cultures, cerebrospinal fluid cultures and cultures from abscesses, catheters, prosthetic materials and other devices were discussed.
The PID consultant reviewed the prescriptions of patients managed with targeted antibiotics/antifungals and held an interview with the attending physician to issue a recommendation on the most appropriate antimicrobial treatment.
Statistical analysisTo describe the study population, we entered information on the selected variables in summary tables with the SPSS (Statistical Package for the Social Sciences) package.20 Categorical variables were presented as frequency and percentages and continuous variables as median and interquartile ranges (IQR). We compared antimicrobial use (DOT, DOT/1000 inpatient days, ABxS and ABxS/1000 admissions) in P1 and P2. We also calculated the differences in the average costs between P1 and P2, expressed in terms of absolute savings and percentage change.
ResultsDuring P2, a total of 160 patients were included in the study. Of this total, 55.6% were male, and most were admitted to the oncology (31.2%), surgery (22.1%) or general paediatric inpatient (10%) wards.
As regards the risk factors for infection, 65.2% of the patients had a relevant underlying disease, such as lymphoma or leukaemia (25.8%), a solid tumour (13.8%) or congenital heart disease (11.9%). Thirty-seven percent of the patients were central-line catheter carriers (Port-a-Cath [57.6%], femoral vein catheter [13%] or peripherally inserted central line [10.8%]).
The PACTA-Ped recommendations given by the PID consultant were as follows: no change to the prescribed antimicrobial treatment in 45% of care episodes, narrowing the antimicrobial spectrum in 35%, discontinuation of any or the prescribed antimicrobials in 6.9%, switch to the oral route in 6.9% and broadening the antimicrobial spectrum in 3.1%. The overall rate of acceptance of these recommendations by the prescribing physician was 89%.
The antibiotics involved most frequently in the recommendations were meropenem (41.6%), cefotaxime (23.4%), ceftazidime (7.4%), vancomycin (6.5%), piperacillin-tazobactam (6.5%) and teicoplanin (5.2%). The antibiotics recommended most frequently as alternatives were ciprofloxacin (11.9%) and gentamicin (11.9%). As regards the reviewed antifungal treatments, the most frequently involved agents were liposomal amphotericin B (60%), fluconazole (25%) and voriconazole (10%). In 83.3% of the antifungal treatment events, the PID consultant agreed with the prescription and did not recommend any changes.
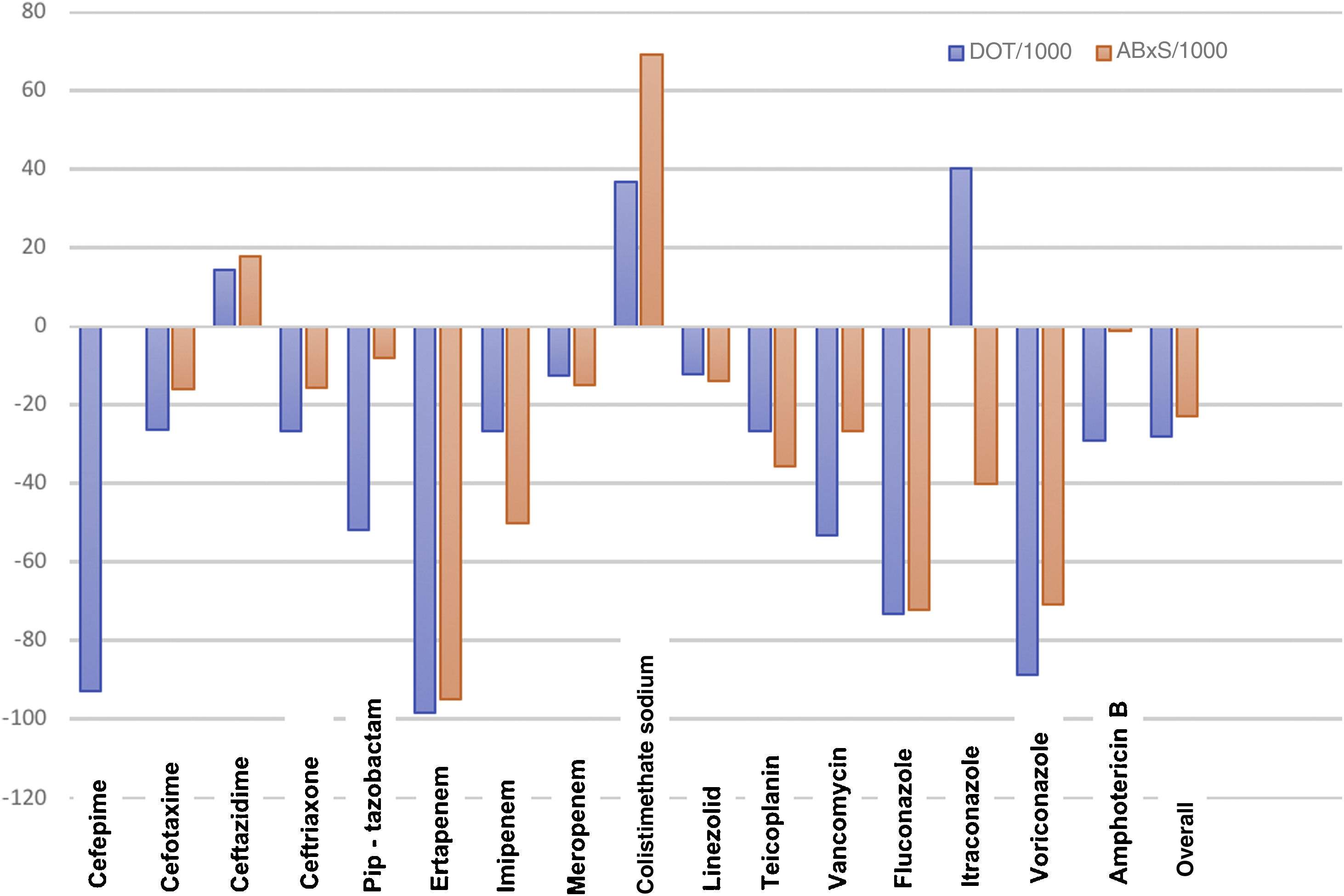
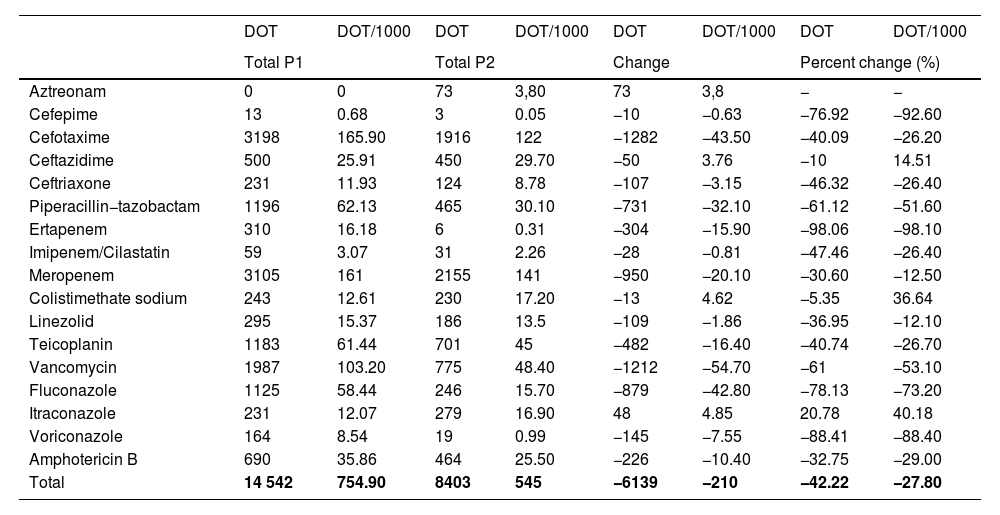
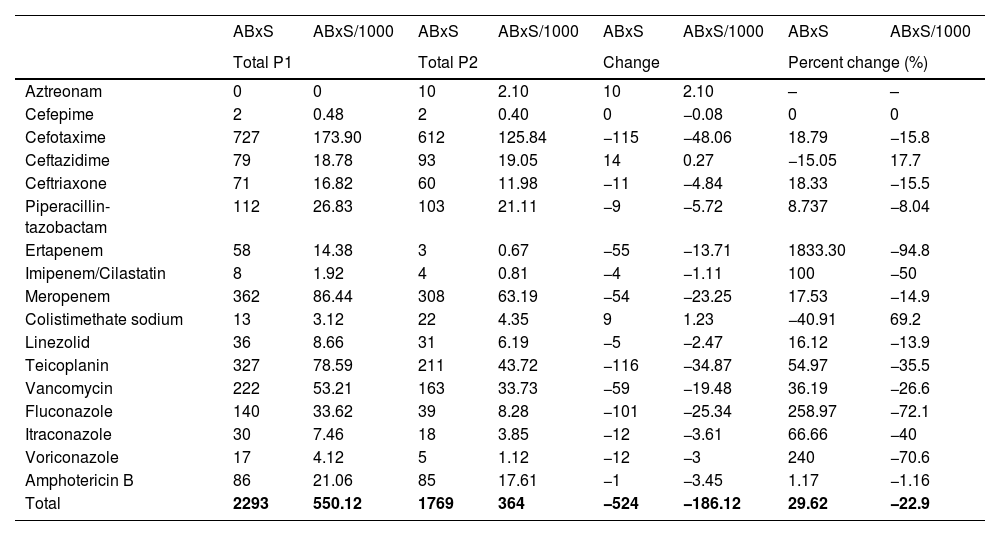
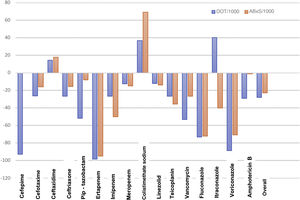
Antimicrobial useTables 1 and 2 summarise the antimicrobial use data for P1 and P2 in terms of the DOT, DOT/1000, ABxS and ABxS/1000. We found an average of decrease of 27.8% in the DOT/1000 inpatient days in P2 after the start of the ASP. We also found an overall decrease of 22.9% in the ABxS/1000 admissions in P2 compared to P1. The use of carbapenems, cephalosporins and glycopeptides decreased in P2 compared to P1 (in terms of the DOT/1000 inpatient days and the ABxS/1000 admissions). Fig. 1 presents the percentage change in the DOT/1000 and ABxS/1000 for each antibiotic or antifungal between the 2 periods. There was an increase in the consumption of ceftazidime and colistin in P2.
Days of therapy and days of therapy per 1000 inpatient days for each targeted antimicrobial in each year period.
| DOT | DOT/1000 | DOT | DOT/1000 | DOT | DOT/1000 | DOT | DOT/1000 | |
|---|---|---|---|---|---|---|---|---|
| Total P1 | Total P2 | Change | Percent change (%) | |||||
| Aztreonam | 0 | 0 | 73 | 3,80 | 73 | 3,8 | − | − |
| Cefepime | 13 | 0.68 | 3 | 0.05 | −10 | −0.63 | −76.92 | −92.60 |
| Cefotaxime | 3198 | 165.90 | 1916 | 122 | −1282 | −43.50 | −40.09 | −26.20 |
| Ceftazidime | 500 | 25.91 | 450 | 29.70 | −50 | 3.76 | −10 | 14.51 |
| Ceftriaxone | 231 | 11.93 | 124 | 8.78 | −107 | −3.15 | −46.32 | −26.40 |
| Piperacillin−tazobactam | 1196 | 62.13 | 465 | 30.10 | −731 | −32.10 | −61.12 | −51.60 |
| Ertapenem | 310 | 16.18 | 6 | 0.31 | −304 | −15.90 | −98.06 | −98.10 |
| Imipenem/Cilastatin | 59 | 3.07 | 31 | 2.26 | −28 | −0.81 | −47.46 | −26.40 |
| Meropenem | 3105 | 161 | 2155 | 141 | −950 | −20.10 | −30.60 | −12.50 |
| Colistimethate sodium | 243 | 12.61 | 230 | 17.20 | −13 | 4.62 | −5.35 | 36.64 |
| Linezolid | 295 | 15.37 | 186 | 13.5 | −109 | −1.86 | −36.95 | −12.10 |
| Teicoplanin | 1183 | 61.44 | 701 | 45 | −482 | −16.40 | −40.74 | −26.70 |
| Vancomycin | 1987 | 103.20 | 775 | 48.40 | −1212 | −54.70 | −61 | −53.10 |
| Fluconazole | 1125 | 58.44 | 246 | 15.70 | −879 | −42.80 | −78.13 | −73.20 |
| Itraconazole | 231 | 12.07 | 279 | 16.90 | 48 | 4.85 | 20.78 | 40.18 |
| Voriconazole | 164 | 8.54 | 19 | 0.99 | −145 | −7.55 | −88.41 | −88.40 |
| Amphotericin B | 690 | 35.86 | 464 | 25.50 | −226 | −10.40 | −32.75 | −29.00 |
| Total | 14 542 | 754.90 | 8403 | 545 | −6139 | −210 | −42.22 | −27.80 |
DOT, days of therapy; DOT/1000, days of therapy per 1000 inpatient days.
Antibiotic treatment starts and starts per 1000 hospital admissions for each antibiotic and each period under study.
| ABxS | ABxS/1000 | ABxS | ABxS/1000 | ABxS | ABxS/1000 | ABxS | ABxS/1000 | |
|---|---|---|---|---|---|---|---|---|
| Total P1 | Total P2 | Change | Percent change (%) | |||||
| Aztreonam | 0 | 0 | 10 | 2.10 | 10 | 2.10 | – | – |
| Cefepime | 2 | 0.48 | 2 | 0.40 | 0 | −0.08 | 0 | 0 |
| Cefotaxime | 727 | 173.90 | 612 | 125.84 | −115 | −48.06 | 18.79 | −15.8 |
| Ceftazidime | 79 | 18.78 | 93 | 19.05 | 14 | 0.27 | −15.05 | 17.7 |
| Ceftriaxone | 71 | 16.82 | 60 | 11.98 | −11 | −4.84 | 18.33 | −15.5 |
| Piperacillin-tazobactam | 112 | 26.83 | 103 | 21.11 | −9 | −5.72 | 8.737 | −8.04 |
| Ertapenem | 58 | 14.38 | 3 | 0.67 | −55 | −13.71 | 1833.30 | −94.8 |
| Imipenem/Cilastatin | 8 | 1.92 | 4 | 0.81 | −4 | −1.11 | 100 | −50 |
| Meropenem | 362 | 86.44 | 308 | 63.19 | −54 | −23.25 | 17.53 | −14.9 |
| Colistimethate sodium | 13 | 3.12 | 22 | 4.35 | 9 | 1.23 | −40.91 | 69.2 |
| Linezolid | 36 | 8.66 | 31 | 6.19 | −5 | −2.47 | 16.12 | −13.9 |
| Teicoplanin | 327 | 78.59 | 211 | 43.72 | −116 | −34.87 | 54.97 | −35.5 |
| Vancomycin | 222 | 53.21 | 163 | 33.73 | −59 | −19.48 | 36.19 | −26.6 |
| Fluconazole | 140 | 33.62 | 39 | 8.28 | −101 | −25.34 | 258.97 | −72.1 |
| Itraconazole | 30 | 7.46 | 18 | 3.85 | −12 | −3.61 | 66.66 | −40 |
| Voriconazole | 17 | 4.12 | 5 | 1.12 | −12 | −3 | 240 | −70.6 |
| Amphotericin B | 86 | 21.06 | 85 | 17.61 | −1 | −3.45 | 1.17 | −1.16 |
| Total | 2293 | 550.12 | 1769 | 364 | −524 | −186.12 | 29.62 | −22.9 |
ABxS, antibiotic starts; ABxS/1000, antibiotic starts per 1000 admissions.
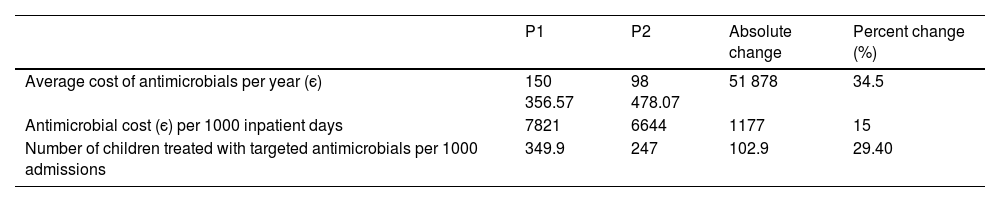
The average annual cost of antimicrobial treatment decreased from є150 356 a year during the P1 to є98 478 a year in P2, which corresponded to average savings of є51 878 per year. When we adjusted the costs for the days of hospitalization, we found antimicrobial treatment costs amounting to є7821/1000 inpatient days in P1 and є6644/1000 inpatient days in P2 (savings of є1177/1000 inpatient days) (Tables 3 and 4). The number of children treated with targeted antimicrobials per 1000 admissions decreased from 349.9/1000 admissions in P1 to 247/1000 admissions in P2, and the overall reduction in the number of children treated with broad-spectrum antibiotics was 102.9/1000 admissions (29.4%) (Table 3).
Average annual cost of antimicrobial treatment per period in euro and percentage change between periods.
| P1 | P2 | Absolute change | Percent change (%) | |
|---|---|---|---|---|
| Average cost of antimicrobials per year (є) | 150 356.57 | 98 478.07 | 51 878 | 34.5 |
| Antimicrobial cost (є) per 1000 inpatient days | 7821 | 6644 | 1177 | 15 |
| Number of children treated with targeted antimicrobials per 1000 admissions | 349.9 | 247 | 102.9 | 29.40 |
The implementation of an ASP in a tertiary care children’s hospital has contributed to reducing the use of broad-spectrum antimicrobials and inappropriate antimicrobial use. Antimicrobial stewardship programmes have been evaluated previously in different care settings in the United States, where clinical practices and prescription patterns differ from those in the EU.6,21–23 In recent years, there reports on the performance of ASPs in paediatric inpatient care settings in Europe have been published.24,25 However, the evidence on the impact of implementing such programmes in paediatric care remains scarce, with limited data published for Europe.26
In our sample, 55% of prescriptions could be improved (“no change” to the prescribed antimicrobial was recommended in only 45% of the episodes), suggesting that strategies need to be implemented to help clinicians improve their antimicrobial prescribing. However, the rate of acceptance of the recommendations made by the stewardship team is one of the highest reported in paediatric studies, with previously reported acceptance rates ranging from 78% to 89%.14,23,25,27–29 The prospective audit in our ASP relies on the one-on-one open dialogue between members from the ASP team and attending physicians.30 In Spanish, the acronym “PACTA” also means “make a deal or agreement”. We feel that this term reflects the spirit of this kind of intervention, which focuses on the dialogue with and education of the prescribers. Paediatric infectious disease consultants have reported that restricting physician autonomy can pose a barrier for the development of ASPs.31 For this reason, the guidance of our team is not of a compulsory nature, but a recommendation agreed on with the prescribing physician. Gross et al. attributed the differences in acceptance rates to the expertise of the medical staff of the ASP and the rapport developed with prescribers.32 It is clear that achieving a high rate of acceptance for the recommendations on antibiotic prescribing requires a close, educational and consensual dialogue with the prescriber.
In the early 1980s, the World Health organization adopted and recommended the use of defined daily doses (DDDs) to standardize and measure antimicrobial use.33 Although it is considered the standard benchmark for adult patients, DDDs cannot be easily applied to measuring antimicrobial use in paediatric patients due to the substantial variation in body weight in this population.34 Our analysis is based on the DOT and ABxS approach, adjusted by 1000 patient inpatient days and 1000 hospital admissions, respectively, which seems more appropriate for the paediatric population and has been supported by different studies.35,36 Other authors have used the DOT/1000 patient days for antimicrobial consumption in paediatric settings.23 The DDD has been used to assess antimicrobial use in some specific subpopulations (neonatology) on account of the narrow range in body weights.37,38 However, the DOT and ABxS adjusted for patient days and number of admissions are accurate markers of antimicrobial use in the paediatric population. We found a decrease in the DOT among targeted antibiotics/antifungals after the start of the ASP. The direct surveillance of antimicrobial prescribing habits can contribute to reducing the inappropriate duration of broad-spectrum antibiotics, with a reduction in the DOT/1000. An increase in the DOT/1000 in alternative antibiotics with a narrower spectrum (like ceftazidime) reflects a switch away from antibiotics with broader spectrum, like meropenem and piperacillin-tazobactam, in P2. There was an increase in the DOT/1000 for colistin in P2; this is an antibiotic with a very limited indication in paediatrics, and only 3 patients per 1000 admissions (ABxS/1000) received this antibiotic in P1 and 4 per 1000 admissions in P2. In spite of this, the presence of a very small number of patients with MDR infections that required prolonged treatment with colistin caused an increase in the DOT/1000.
Meropenem was the antibiotic for which the most recommendations were made. Increased carbapenem use in a hospital is associated with a higher risk of infection by carbapenem resistant bacteria (CRB).39 Carbapenem resistance is a very concerning and growing problem in the US and in Europe, and actions against the inappropriate use of carbapenems should be taken in all settings.7
We also found an overall decrease in the ABxS in P2 after the start of the ASP. The personal interview with the prescriber was a good educational opportunity, because physicians learnt from their own prescribing habits. The decrease in the ABxS may be explained by the change in prescribing habits derived from the direct educational activities included in the ASP, but the increased awareness of health institutions regarding the problem of AMR may also have contributed to it.
Third generation cephalosporins are other targeted antibiotics whose use underwent a marked decrease (26%). The inappropriate use of cephalosporins has been associated with the development of drug resistance in bacteria.4 The presence of broad-spectrum beta-lactamase producing bacteria has become a growing concern in paediatric hospitals in the past few years.4
In US settings, vancomycin is usually the antibiotic for which most recommendations are performed in ASPs.23,27 Given its importance, Di Pentima et al. implemented a specific ASP focused on vancomycin use in a paediatric teaching hospital.23,27 In our study, only 6.5% of all recommendations in the ASP concerned vancomycin. The prevalence of oxacillin-resistant Staphylococcus aureus in the paediatric population in Spain is lower compared to the US, and this fact can explain differences in vancomycin prescribing habits between countries. In any case, after the start of the ASP program, there was a reduction in the prescribing of vancomycin of up to 53% in terms of the DOT/1000.
We observed a steep decrease in antifungal prescribing in P2. There was a decrease in the number of antifungal treatments started in P2 (ABxS/1000) and in the length of treatment (DOT/1000) for voriconazole, fluconazole and liposomal amphotericin B. The consumption of itraconazole decreased in terms of the ABxS/1000 but increased in terms of the DOT/1000 during P2. This difference was associated with a patient admitted in P2 with an invasive fungal infection that required very prolonged treatment with itraconazole.
Overall, there was a progressive reduction in the use of broad-spectrum antibiotics, from 349.9 patients treated with targeted antibiotics per 1000 admissions in P1 to 247 in P2. This decreasing trend in antimicrobial use started in 2015, before the PACTA-Ped program, so antibiotic prescribing may be influenced by other factors. Before 2016, the PID unit introduced a protocol for monitoring children with positive cultures (blood, cerebral spinal fluid) every day, holding daily meetings with the microbiology department and issuing treatment recommendations for those patients. The meetings have continued past 2016 to today. We feel it is of utmost importance to maintain a daily interaction with the department of microbiology. This culture of rapid response after a positive culture result guides clinicians in therapeutic decision-making, contributes to the rapid de-escalation of antimicrobial treatment and reduces inappropriate antimicrobial use, and should be a cornerstone of any ASP. On this note, we want to underscore that the strategy of combining prospective audits with the attending physician and daily meetings with department of microbiology facilitated a progressively decreasing trend in the consumption of targeted antimicrobials in the sample under study.
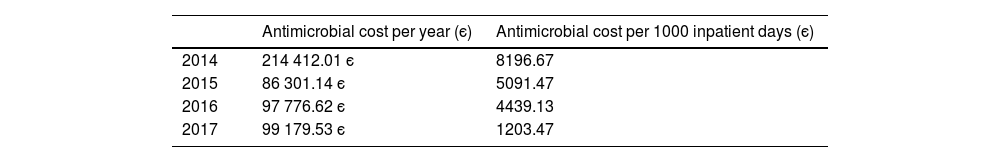
In line with the reduction of the use of broad-spectrum antibiotic in P2, there was a decrease in the average annual cost of antimicrobial treatment, from є150 356 in P1 to є98 478 in P2 (savings of є51 878). This corresponds to a reduction of 34.5% in the cost associated to antimicrobial treatment per year in the inpatient wards. There was also a reduction in the cost of antimicrobial treatment per 1000 inpatient days from є7821 to є6644 euros (reduction of 15%). It is interesting to underline that before the implementation of PACTA-Ped program, there was a cost reduction from 2014 to 2015 in antimicrobial costs per 1000 inpatient days (from є8196 to є5091). As we noted above, this fact suggests that in addition to the PACTA-Ped program itself, other strategies to improve antimicrobial prescription prior to this programme, such as the daily meetings with the department of microbiology, contribute very positively to reducing antimicrobial consumption.
The cost analysis did not include patients managed in the paediatric or neonatal intensive care units or in the emergency department because these settings were not included in the electronic prescription system at the time of the study. The potential savings may be even greater in the future once those units are included in de PACTA-Ped programme. In this ASP, a PID consultant devoted 2 days a week to reviewing the prescriptions and conducting the interviews with physicians, which carried a cost of є24 000 per year for the hospital. In our centre, the ASP seems to be a cost-effective measure, although more studies are required to asses the cost-effectiveness of our ASP over a longer period.
There are several limitations to our study. First at all, there are no standardized metrics to measure antimicrobial use in children. As noted above, the DDD, which is the standard measure in adults, is not suitable for application in the paediatric population without adjustments. Despite the limited experience with the DOT/1000 and the ABxS/1000, we deemed them simple and reliable indicators of antimicrobial use in children, and easy to apply in paediatric ASPs. Another limitation that we did not evaluate other variables that may have contributed to the observed decrease in broad-spectrum antimicrobial use besides the ASP. It is unlikely that the complexity of the patients decreased in P2, as there were no relevant changes in the activity of our hospital. Other educational activities on AMR and antimicrobial use have been implemented in the hospital (lectures, research works). The increased awareness among prescribers could have contributed to the decrease in the inappropriate use of antimicrobials, independently of the ASP. An ASP is a bundle of actions aimed at improving the prescribing culture with a long-term effect. It is very challenging to measure which parts of the ASP are responsible for the overall result, so maybe the PACTA-Ped has a lower impact than we have attributed to it. However, the ASP is a programme combining different actions, and its results should be evaluated as a whole.
To conclude, an ASP based on periodic reviews of antimicrobial prescriptions and one-to-one audits with prescribers achieved a significant decrease in the use of broad-spectrum antibiotics and antifungals in a tertiary care paediatric hospital. The costs associated with antimicrobial prescribing decreased since the beginning of the ASP, which was a cost-effective action in the period under study.
FundingThis work was supported by the Spanish Ministry of Science and Innovation-Instituto de Salud Carlos III and the European Regional Development Fund (ERDF) (Río Hortega contracts CM20/00173 [SV] and INT20/00086 [DBG]) and by Instituto de Investigación Hospital 12 de Octubre (imas12).
Ethical considerationsThe study was approved by the Scientific Committee of Hospital Universitario 12 de Octubre (code: TP17/0330).
Conflict of interestSV, JMC, SD, MAO and PR have no conflicts of interest to declare.
DBG has received fees from MSD as a speaker in educational activities.
CE has received grants to attend scientific meetings from Viiv and Gilead.
Availability of data and materialsThe data were hosted in a secure online server located at Instituto de Investigación Hospital 12 de Octubre. The datasets used and analysed during the current study are available from the corresponding author upon reasonable request. Code availability: https://redcap.imas12.es.
Author contributionsSerena Villaverde and Daniel Blázquez-Gamero contributed to the study conception and design. Daniel Blázquez-Gamero and José Manuel Caro prepared the materials, collected the data and performed the analysis. Serena Villaverde wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Luis Prieto, Cinta Moraleda, Ángela Manzanares, Elisa Fernández-Cooke, María Fernanda Guzmán, David Lora, Cristofer Izquierdo and Francisco López-Medrano.