To evaluate a telephone support programme for mothers who breastfeed for the first 6 months.
MethodsA randomised unmasked clinical trial was conducted in 5 urban Primary Care centres that included mothers with healthy newborns who were breastfeeding exclusively (EBF) or partially (PBF). The control group received the usual care. The intervention group also received telephone support for breastfeeding on a weekly basis for the first 2months and then every 2weeks until the sixth month. The type of breastfeeding was recorded in the usual check-up visit (1, 2, 4 and 6 months).
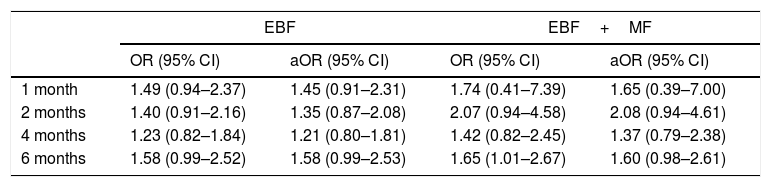
ResultsThe study included 193 patients in the intervention group, and 187 in a control group. The greatest increase in the percentage of EBF was observed at 6 months: 21.4% in the control group compared to 30.1% in the intervention group. However, in the adjusted odds ratios analysis, confidence intervals did not show statistical significance. The odds ratio at 1 month, 2 months, 4 months, and 6 months for EBF were 1.45 (0.91–2.31), 1.35 (0.87–2.08), 1.21 (0.80–1.81), and 1.58 (0.99–2.53), respectively. The odds ratio in the same age groups for any type of breastfeeding (EBF+PBF) were 1.65 (0.39–7.00), 2.08 (0.94–4.61), 1.37 (0.79–2.38), and 1.60 (0.98–2.61), respectively.
ConclusionsTelephone intervention was not effective enough to generalise it.
Evaluar un programa de apoyo telefónico a madres que dan lactancia materna los 6 primeros meses.
MétodosEnsayo clínico aleatorizado no enmascarado. Colaboraron 5 centros de salud de medio urbano. Se incluyeron madres con recién nacidos sanos que tomaban lactancia materna exclusiva (LME) o parcial (LMP). El grupo control recibió la atención habitual. El grupo intervención recibió además apoyo telefónico semanal los 2primeros meses y quincenal hasta el sexto mes. Se valoró el tipo de lactancia en las revisiones habituales (1, 2, 4 y 6 meses).
ResultadosGrupo intervención n = 193, grupo control n = 187. La mayor diferencia en porcentaje de LME se apreció a los 6 meses: 21,4% de grupo control frente al 30,1% del grupo intervención. No obstante, en el análisis ajustado de las odds ratio los intervalos de confianza no mostraron significación estadística. Las odds ratio al mes, 2 meses, 4 meses y 6 meses para LME fueron respectivamente: 1,45 (0,91-2,31); 1,35 (0,87-2,08); 1,21 (0,80-1,81) y 1,58 (0,99-2,53). Las odds ratio en los mismos cortes para cualquier tipo de lactancia materna (LME+LMP) fueron: 1,65 (0,39-7,00); 2,08 (0,94-4,61); 1,37 (0,79-2,38) y 1,60 (0,98-2,61).
ConclusionesLa intervención telefónica no fue suficientemente efectiva como para generalizarla.
Breastfeeding is the natural way to feed infants and exclusive breastfeeding is recommended through the first 6 months of life.1 There is ample evidence of its benefits for infants and mothers,2 and also evidence of the association of formula feeding with several health problems.3 Few countries achieve adequate proportions of exclusive breastfeeding (EBF) at 6 months of life. In developed countries, there has been a recent increase in the rate of initiation of EBF, but this is followed by a progressive decline through time, with very low percentages of EBF at 6 months.4 According to the latest National Health Survey, the prevalence of EBF at 6 months in Spain is 28.5%.5
The reasons for these very low percentages are varied, and often involve issues that are well outside the scope of our health care or health promotion activity. A series of risk factors for discontinuation of breastfeeding have been described in the literature, such as membership in specific ethnic groups, teen motherhood, single-parent household or caesarean delivery.3,6,7 Being aware of these risk factors allows health care professionals to be more alert when it comes to supporting EBF in these groups.
Multiple strategies have been tried in the general population and in at-risk groups to improve successful breastfeeding rates. The first days after birth are crucial and are the time when the awareness and training of professionals involved in maternity care is most important.8 After discharge, a long road starts during which quality information and support can ultimately determine the failure or success of EBF. A broad variety of strategies to promote breastfeeding have been tested, and many of them are beneficial.7,9–11 A proactive attitude7 and intervening as soon as possible12–14 are important factors. The support strategies that have been tried include support by health care professionals (especially midwives15 or nurses)16 and support by groups outside the health care system,17,18 and both types of intervention have proven useful.7,9 There is evidence that face-to-face support can be helpful,7 while studies on telephone-based support have shown favourable results in some instances,16,19–22 but found no clear effect in others.23–25 The combined use of several of these interventions enhances their effects and leads to better outcomes.12,26
Few studies on the promotion of EBF have been conducted in Spain.27 The studies we found28,29 showed that breastfeeding interventions were beneficial, although there were no clinical trials on the subject. The characteristics of our health care system, with paediatricians and paediatric nurses working in primary care settings, offer optimal conditions for the promotion of EBF and make it possible to implement universal interventions. For this reason, we decided to evaluate a telephone-based support programme delivered by paediatric nurses in the primary care system for mothers that were breastfeeding their children the first 6 months of life with the aim of improving the duration of breastfeeding.
Materials and methodsStudy design and periodWe conducted a multicentre randomised clinical trial. The intervention and data collection took place between October 2014 and October 2016.
SettingWe conducted the study in 3 primary care centres in Cornellà de Llobregat (Sant Ildefons, Jaume Soler and Gavarra), 2 in Esplugues de Llobregat (Can Vidalet and Lluís Millet) and 1 in Sant Boi (Montclar). All participating nurses had some degree of training on breastfeeding, were employed as primary care paediatric nurses and had at least 1 year of experience. Generally speaking, participants were residents of low-to-medium income urban neighbourhoods in the Barcelona metropolitan area.
Inclusion criteriaMothers of healthy infants delivered at term (≥37 weeks) in the catchment population of participating centres and who were breastfeeding their children (exclusive or partial breastfeeding).
Exclusion criteriaAdmission of infant or mother to ICU, multiple pregnancy, severe congenital malformation in the infant, maternal age of 18 years or less, or mother that lacked a phone or had a language barrier.
Sample sizeIn our region, the prevalence of EBF at 6 months of age is of approximately 32%. Similar studies in developed countries have found a relative risk of improvement in EBF post intervention of 1.44.9 We used the !NSize macro for SPSS30 to calculate the sample size for a 1:1 ratio between groups, a 5% probability of a type I error and a 20% probability of a type II error. The resulting size was 376 mothers (188 in each arm). We estimated a dropout rate of 10%, so we sought to recruit a final sample of 414 participants.
Dependent variables1: Exclusive breastfeeding (EBF), infant fed exclusively with human milk. 2: Mixed feeding (MF), infant fed any amount of formula or other type of foods in addition to breastfeeding. 3: Formula feeding (FF), infant not fed any human milk. We assessed the type of feeding based on the criteria established by the WHO: food received by the infant in the past 24h.
Independent variablesMaternal age, place of birth, maternal educational attainment, smoking, type of household, help from grandparents in child care, sibling order, previous experience breastfeeding, attendance to childbirth workshop, contact with some type of breastfeeding support group, date of maternal return to work during the study (if mother started working), prenatal care, disease that required hospital admission during pregnancy (if applicable), gestational age, type of delivery, Apgar at 1 and 5minutes, birth weight, and type of feeding at the time of the first visit.
Method of recruitment and group assignmentRecruitment and assignment were performed by the paediatrician during the first visit of the infant to the primary care centre. The paediatrician assessed the inclusion criteria in all mothers of infants born in the period under study and invited them to participate in the study if they met all the criteria. The mothers that agreed to participate were assigned to the experimental or the control group using a random number table generated by computer software after signing an informed consent form.
Data collection and followupDemographic data and information on the pregnancy and delivery were collected during the first visit of the infant. During the followup, at each of the visits programmed in the framework of the Protocol of Preventive and Health Promotion Activities in the Paediatric Age Group (at 1, 2, 4 and 6 months of life), the professional that managed the visit recorded whether the infant was receiving EBF, MF or FF.
Description of the interventionBefore starting the study, we held a meeting with the nurses in each primary care centre to ensure they provided consistent information and advice to mothers according to a protocol that we will explain in more detail.
Mothers in both the control and the experimental groups attended the visits included in the preventive care protocol: an initial postnatal visit with the paediatrician (between days 7 and 15 post birth), and checkups at 1, 2, 4 and 6 months with the nurse assigned to the patient, under the supervision of the paediatrician. The nurse, as is customary in our primary care clinics, was the professional in charge of counselling the mother regarding nutrition during these visits. Mothers were offered the option of scheduling additional appointments or calling the nurse on the phone to receive guidance regarding breastfeeding problems. In addition to having the same routine visits as the control group, mothers assigned to the experimental group received a weekly call during the first 2 months and a call every other week between months 2 and 6 post birth. The nurse assigned to the infant made the calls. Likewise, the specific nurse that managed the face-to-face visits with the mother was the one that provided support over the phone.
Given the difficulty of structuring this type of interview, we established basic themes based on the age of the infants that we considered important to address in the telephone-based intervention. During the first month: position of the newborn for breastfeeding, frequency of feeds, number and consistency of stools, general breast care, normal weight gain, and, in mothers that supplemented feedings, advice and support to try to re-establish EBF. In months 2–3: advice on expressing breast milk to have stores in case the mother returned to work or ever needed to be away from home, with instructions on how to handle and store breast milk. In months 4–6: how to use stored breast milk (if any was stored) and techniques on the administration of stored milk to infants, importance of maintaining EBF and avoiding administration of other types of milk or foods.
If any mother in the intervention group stopped breastfeeding completely, she stopped receiving these calls.
Statistical analysisWe performed a descriptive analysis of the variables and assessed for potential differences between the control and experimental groups. We summarised quantitative variables using the mean and compared them by means of the Student t test, and summarised categorical variables as proportions and compared them by means of the chi square test. We calculated crude odds ratios (ORs) with their corresponding 95% confidence intervals comparing the prevalence of EBF (versus MF+FF) and of any breastfeeding (EBF+MF vs FF) in the experimental and control groups. We made these comparisons at 1, 2, 4 and 6 months. We took into account the possibility that some variables could act as confounders and affect the ORs. To assess this possibility, we used the strategy proposed by Maldonado and Greenland.31 We included potential confounders in a logistic regression model to obtain the adjusted odds ratios. We also calculated the absolute risk reduction (ARR) for EBF or any breastfeeding (EBF+MF) at each of the ages under study.
We performed an intention to treat analysis using the software SPSS version 19.0.
Ethical considerationsThe study adhered to the principles of the Declaration of Helsinki and to current law (Royal Decree 223/2004 on clinical trials, Law 14/2007 on biomedical research and Law 15/1999 on the protection of personal data).
The study protocol was reviewed by the Research Ethics Committee of the Institut Universitari d’Investigació en Atenció Primària Jordi Gol, which approved the study under file number P14/047.
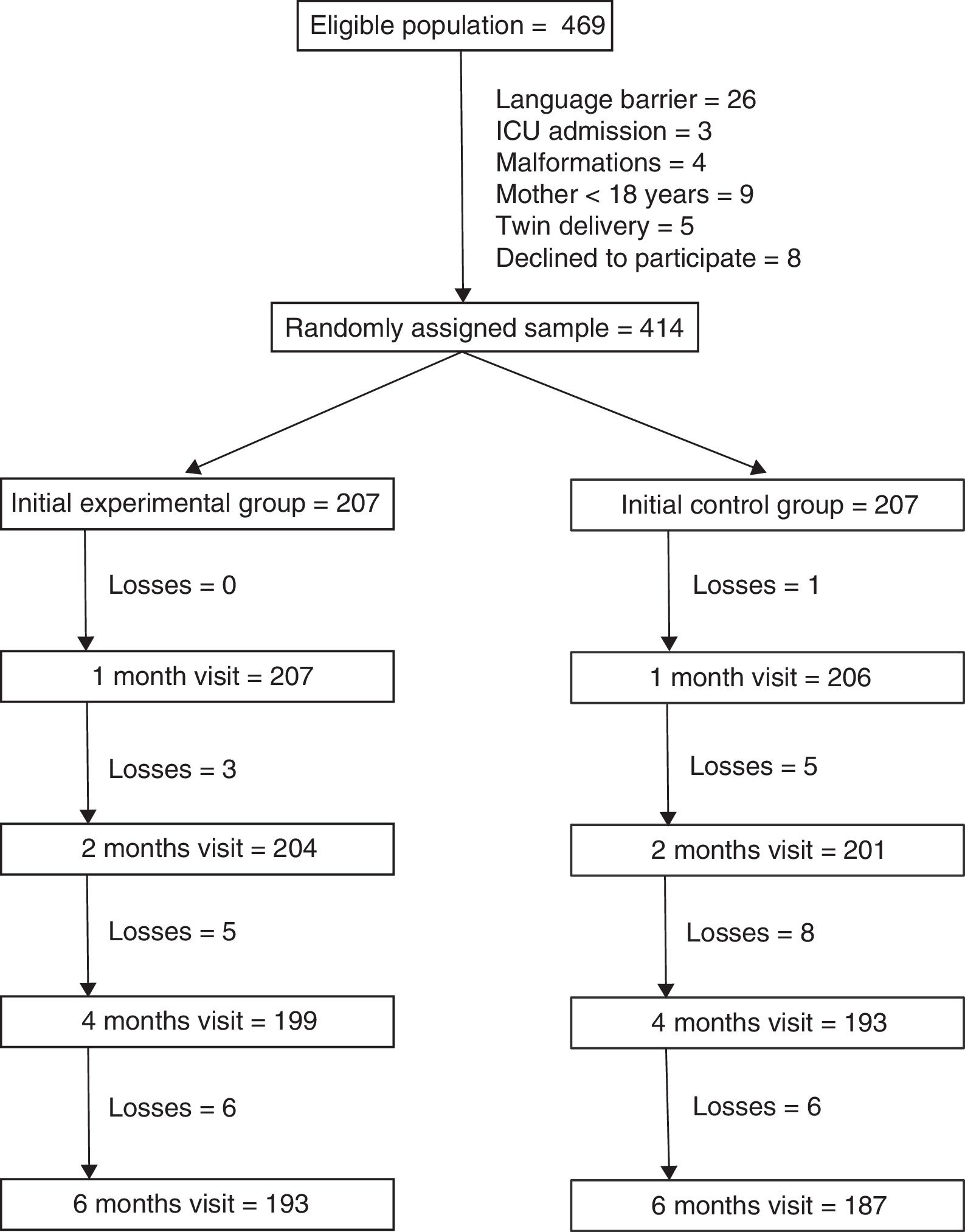
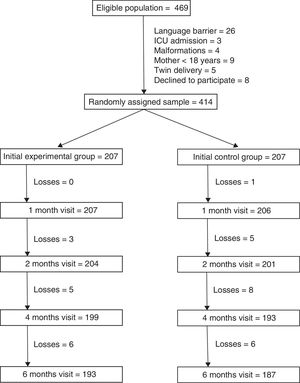
ResultsThere were 469 mothers that met the inclusion criteria. The main reason for exclusion was the presence of a language barrier (5.5% of eligible mothers). We randomly assigned the remaining 414 mothers to the 2 study groups (207 per group). A total of 193 mothers in the intervention group (93.2%) and 187 in the control group (90.3%) completed the 6 months of followup (Fig. 1). Losses to followup were mainly due to changes in residence of the patients that were accompanied by a change of primary care centre. One mother in the intervention group chose to leave the study and 2 mothers were withdrawn because they could not be reached by telephone.
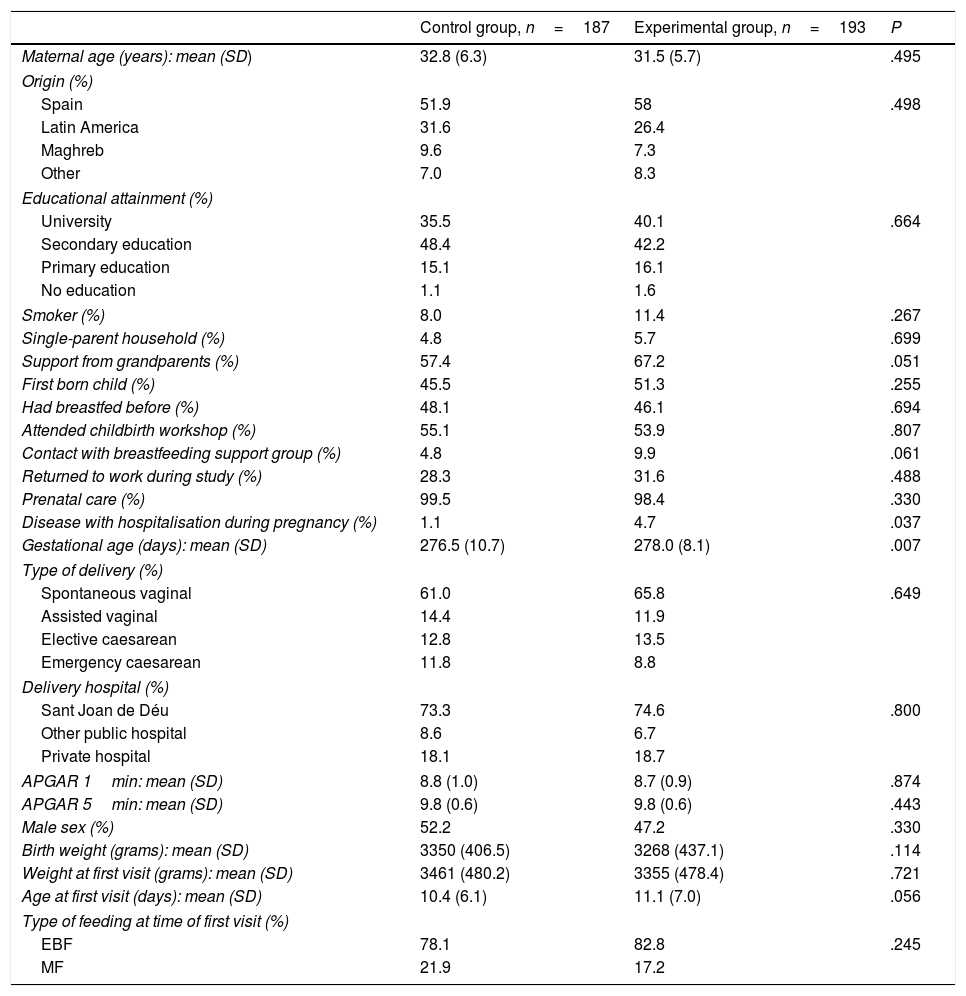
Table 1 compares the characteristics of the 2 groups under study.
Characteristics of the experimental and control groups.
| Control group, n=187 | Experimental group, n=193 | P | |
|---|---|---|---|
| Maternal age (years): mean (SD) | 32.8 (6.3) | 31.5 (5.7) | .495 |
| Origin (%) | |||
| Spain | 51.9 | 58 | .498 |
| Latin America | 31.6 | 26.4 | |
| Maghreb | 9.6 | 7.3 | |
| Other | 7.0 | 8.3 | |
| Educational attainment (%) | |||
| University | 35.5 | 40.1 | .664 |
| Secondary education | 48.4 | 42.2 | |
| Primary education | 15.1 | 16.1 | |
| No education | 1.1 | 1.6 | |
| Smoker (%) | 8.0 | 11.4 | .267 |
| Single-parent household (%) | 4.8 | 5.7 | .699 |
| Support from grandparents (%) | 57.4 | 67.2 | .051 |
| First born child (%) | 45.5 | 51.3 | .255 |
| Had breastfed before (%) | 48.1 | 46.1 | .694 |
| Attended childbirth workshop (%) | 55.1 | 53.9 | .807 |
| Contact with breastfeeding support group (%) | 4.8 | 9.9 | .061 |
| Returned to work during study (%) | 28.3 | 31.6 | .488 |
| Prenatal care (%) | 99.5 | 98.4 | .330 |
| Disease with hospitalisation during pregnancy (%) | 1.1 | 4.7 | .037 |
| Gestational age (days): mean (SD) | 276.5 (10.7) | 278.0 (8.1) | .007 |
| Type of delivery (%) | |||
| Spontaneous vaginal | 61.0 | 65.8 | .649 |
| Assisted vaginal | 14.4 | 11.9 | |
| Elective caesarean | 12.8 | 13.5 | |
| Emergency caesarean | 11.8 | 8.8 | |
| Delivery hospital (%) | |||
| Sant Joan de Déu | 73.3 | 74.6 | .800 |
| Other public hospital | 8.6 | 6.7 | |
| Private hospital | 18.1 | 18.7 | |
| APGAR 1min: mean (SD) | 8.8 (1.0) | 8.7 (0.9) | .874 |
| APGAR 5min: mean (SD) | 9.8 (0.6) | 9.8 (0.6) | .443 |
| Male sex (%) | 52.2 | 47.2 | .330 |
| Birth weight (grams): mean (SD) | 3350 (406.5) | 3268 (437.1) | .114 |
| Weight at first visit (grams): mean (SD) | 3461 (480.2) | 3355 (478.4) | .721 |
| Age at first visit (days): mean (SD) | 10.4 (6.1) | 11.1 (7.0) | .056 |
| Type of feeding at time of first visit (%) | |||
| EBF | 78.1 | 82.8 | .245 |
| MF | 21.9 | 17.2 | |
EBF, exclusive breastfeeding; MF, mixed feeding; SD, standard deviation.
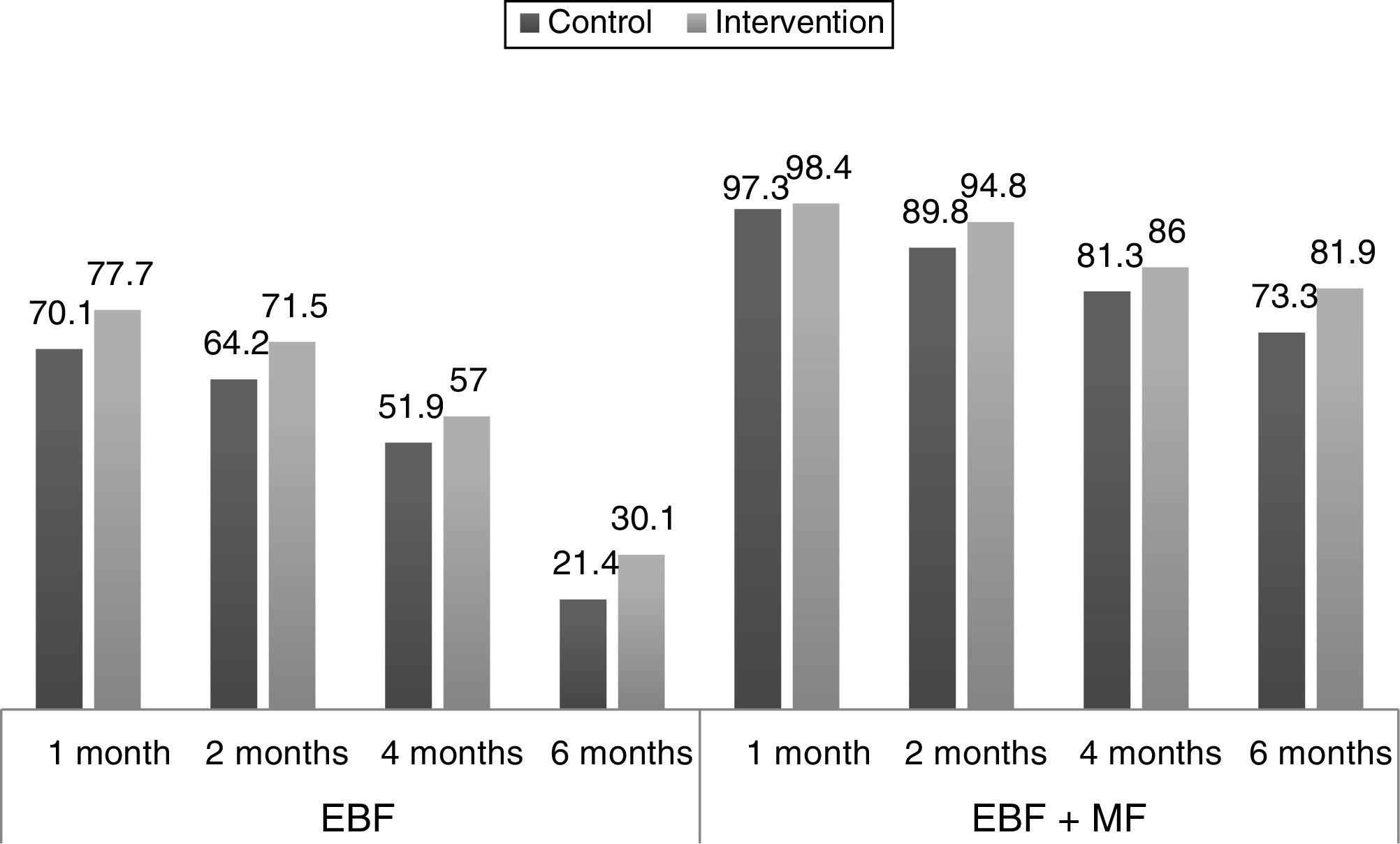
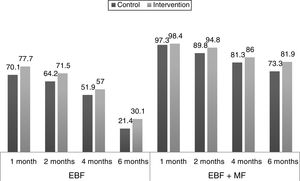
Fig. 2 shows the proportions of the different types of feeding by age in the experimental and the control group, and their changes in the 6 months of followup.
After collecting the data, and using the method mentioned above, we assessed which of the variables could influence the effect of the intervention and ought to be included in the regression model. The only variable that met these conditions was “contact with a breastfeeding support group” (results not shown).
Table 2 presents the crude and adjusted ORs. We compare the ORs in the experimental group to those of the control group for EBF and for any type of breastfeeding (EBF+MF).
Impact of intervention on breastfeeding.
| EBF | EBF+MF | |||
|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | |
| 1 month | 1.49 (0.94–2.37) | 1.45 (0.91–2.31) | 1.74 (0.41–7.39) | 1.65 (0.39–7.00) |
| 2 months | 1.40 (0.91–2.16) | 1.35 (0.87–2.08) | 2.07 (0.94–4.58) | 2.08 (0.94–4.61) |
| 4 months | 1.23 (0.82–1.84) | 1.21 (0.80–1.81) | 1.42 (0.82–2.45) | 1.37 (0.79–2.38) |
| 6 months | 1.58 (0.99–2.52) | 1.58 (0.99–2.53) | 1.65 (1.01–2.67) | 1.60 (0.98–2.61) |
aOR, adjusted odds ratio; CI, confidence interval; EBF, exclusive breastfeeding; MF, mixed feeding; OR, crude odds ratio.
Adjusted for the variable “contact with breastfeeding support group”.
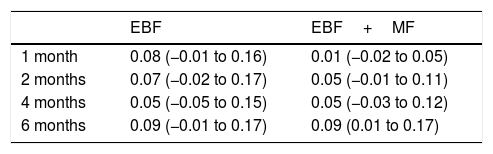
Last of all, Table 3 presents the ARRs for EBF and for any type of breastfeeding (EBF+MF) for each infant age.
Absolute risk reduction (95% CI).
| EBF | EBF+MF | |
|---|---|---|
| 1 month | 0.08 (−0.01 to 0.16) | 0.01 (−0.02 to 0.05) |
| 2 months | 0.07 (−0.02 to 0.17) | 0.05 (−0.01 to 0.11) |
| 4 months | 0.05 (−0.05 to 0.15) | 0.05 (−0.03 to 0.12) |
| 6 months | 0.09 (−0.01 to 0.17) | 0.09 (0.01 to 0.17) |
CI, confidence interval; EBF, exclusive breastfeeding; MF, mixed feeding.
The percentages of breastfeeding in all age groups were higher in the experimental group compared to the control group, but the adjusted ORs and their confidence intervals did not reflect a clear effect of the intervention. Our intervention did not have the expected results for either EBF or any breastfeeding (EBF+MF). Most similar studies in the literature have found a positive effect of the interventions, at least in the early months of life, with subsequent dampening.13,17,19 This was not the case in our study. Indeed, differences in parenting styles and social and cultural factors may result in one intervention having different effects in different places.7,19 Generalising the results of studies on health education, in general and on breastfeeding in particular, is challenging precisely because outcomes are influenced by all of these factors.
It would be hard to determine why our intervention did not have the desired effect. Due to the substantial accessibility of primary care services, mothers that are truly motivated to breastfeed and experience problems may take the initiative to seek the help of the paediatrician or nurse to try to resolve them. There is no way of knowing what happens when mothers do need help but lack the ability to seek it and need health professionals to be proactive instead. It is in this regard that we should have seen the difference in the outcomes of our study. And it seems that in this case, the effect of the intervention on these mothers was not strong enough.
The obtained ARRs were low, and their confidence intervals also indicated that the intervention was not effective. Taking into account that each mother received approximately 15 calls in the first 6 months post birth, and the time invested by the nursing staff on this activity, we needed to obtain much higher ARRs to consider the intervention worth implementing.
Previous studies have shown that the simultaneous combination of different interventions for breastfeeding promotion can enhance their effects.7,10 Thus, while the results of our study were not significant, it is possible that our intervention combined with another breastfeeding promotion strategy would be beneficial. Furthermore, certain interventions have proven effective on population subsets with specific social, ethnic or economic characteristics,17,18,24 so it is also possible that while our intervention had no effect on the general population, it may have been effective in specific population subsets. However, confirming these possibilities would require further research.
The main limitation in our study was the impossibility of masking the intervention. The intervention was designed for implementation in the primary care setting, of which one of the main advantages is the mutual knowledge and trust that can develop between users and health care workers. For this reason, we believed it would be best for the nurse assigned to the patient to be the professional delivering the intervention, with the aim of reinforcing this rapport and help further motivate mothers.
Another limitation in our study was that we found a percentage of EBF at 6 months that was lower than expected. We calculated the sample size based on the 32% of EBF observed in recent years in our area at 6 months post birth. Surprisingly, we found a percentage of EBF at 6 months of only 21.4% in the control group. Some external factor must have been at play to make this percentage decline this much in the past few years. With the number of patients included in the study, we only had a power of 67% in assessing whether the observed increase was significant. A larger sample would have allowed us to obtain more information with the rates of EBF that we finally obtained through the intervention. In any case, studies similar to our own that found improvements in the proportion of EBF reported increases of at least 10% in the experimental group,13,17,19 and we did not obtain increases this high in any age group.
Thus, we concluded that the telephone-based intervention implemented in our study did not have a large enough impact to be considered a useful measure worth implementing universally for improvement of breastfeeding rates.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the mothers that participated in the study, the paediatric nurses that devoted themselves to the project disinterestedly, and the participating paediatricians.
Please cite this article as: Balaguer Martínez JV, Valcarce Pérez I, Esquivel Ojeda JN, Hernández Gil A, Martín Jiménez AP, Bernad Albareda M. Apoyo telefónico de la lactancia materna desde Atención Primaria: ensayo clínico aleatorizado y multicéntrico. An Pediatr (Barc). 2018;89:344–351.
Previous presentations: The results of this study were presented at the 31 National Congress of Outpatient Paediatrics and Primary Care of the Sociedad de Pediatría Extrahospitalaria y Atención Primaria (SEPEAP); October 19–21, 2017; Santander, Spain.