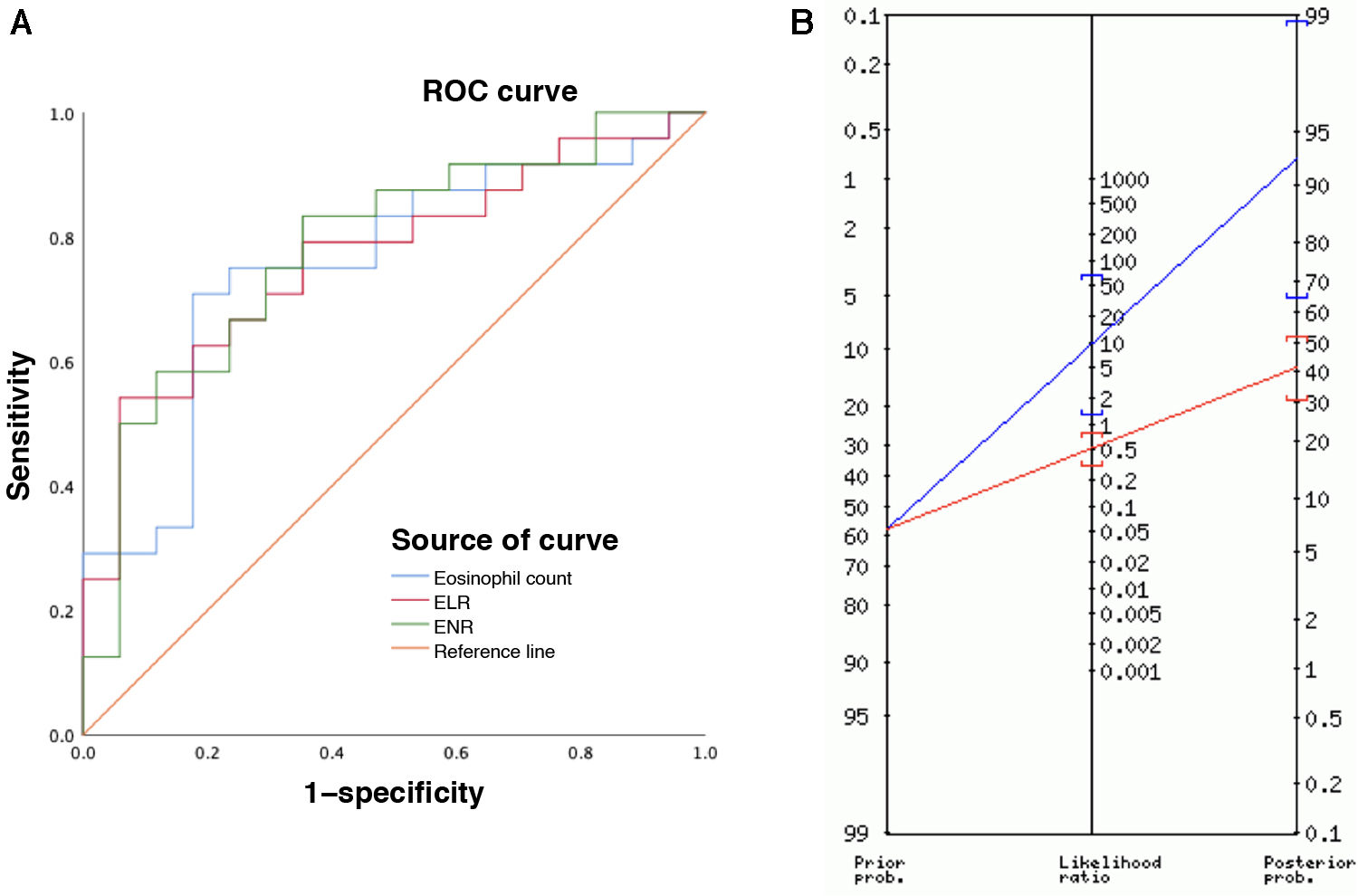
Eosinophilic esophagitis (EoE) is an immune disorder of the esophagus with a predominantly eosinophilic inflammatory response.1 Esophageal endoscopy and biopsy is the method of choice for diagnosis and follow-up, but its invasive nature, considerable cost and potential complications are an ongoing concern.2 Although research has been conducted to determine diagnostic and prognostic value of non-invasive measures, such as eosinophil counts in peripheral blood, the results have been heterogeneous and the evidence to date is insufficient.3 Recent studies have demonstrated the usefulness of cell ratios for various gastrointestinal diseases.4 These ratios can be calculated easily from complete blood count results, thus at a low cost, and, to our knowledge, their value in EoE has yet to be investigated. We assessed the usefulness of cell ratios derived from eosinophil counts in EoE through the diagnostic evaluation of patients aged less than 15 years who underwent an upper GI endoscopy for suspected EoE between 2015 and 2022 in a children’s hospital, including those with normal histology and those with a histological diagnosis of EoE (ID 3318-0000206). All patients presented with suspected EoE, defined by the presence of symptoms of esophageal dysfunction. We analyzed clinical and laboratory variables, and peripheral blood samples were processed with the DxH 900 analyzer (Beckman Coulter, Miami, FL, USA). We calculated the eosinophil-to-lymphocyte ratio (ELR) and the eosinophil-to-neutrophil ratio (ENR) by dividing the eosinophil count by the lymphocyte or neutrophil count, as applicable. The statistical analysis was performed with the software SPSS (IBM Corp., Armonk, NY, USA) with comparative tests for qualitative variables (χ2 or Fisher exact test) and quantitative variables (t test or Mann-Whitney U test) and generation of receiver operating characteristic (ROC) curves with calculation of the area under the curve (AUC) to assess the diagnostic yield of each parameter. The sample included 41 children: 24 with EoE and 17 with normal biopsy results (no EoE). Eosinophil counts and the ELRs and ENRs were higher in patients with EoE compared to patients with no EoE: 673 cells/mm3 vs 346 cells/mm3; 0.2457 vs 0.1253 and 0.3024 vs 0.1225, respectively (P < .05). There were no significant differences in the prevalence of food allergy (no EoE, 29% vs EoE, 58%; P = .067). With respect to the diagnostic yield of the biomarkers, the sensitivity, specificity and PPV for diagnosis of EoE were 70%, 82% and 85% for the eosinophil count, 54%, 94% and 92% for the ELR and 83%, 64% and 76% for the ENR, respectively (Table 1). The marker that offered the highest accuracy was the ELR, with a cut-off point of 0.243 and an odds ratio (OR) of 18.9 (Fig. 1A).
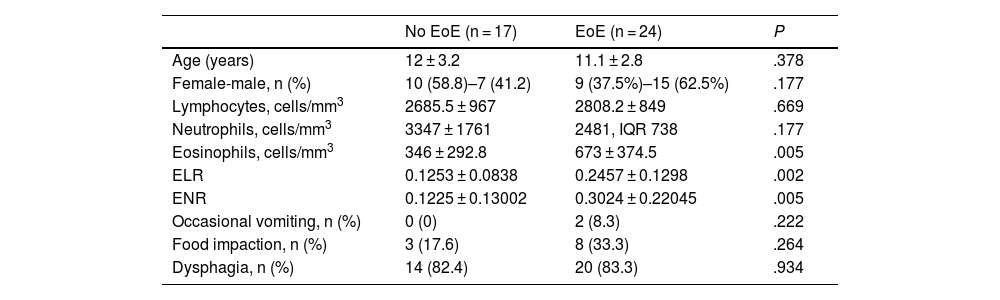
Demographic and Laboratory Characteristics of the Sample. Diagnostic Performance of the Analyzed Parameters.
| No EoE (n = 17) | EoE (n = 24) | P | |
|---|---|---|---|
| Age (years) | 12 ± 3.2 | 11.1 ± 2.8 | .378 |
| Female-male, n (%) | 10 (58.8)–7 (41.2) | 9 (37.5%)–15 (62.5%) | .177 |
| Lymphocytes, cells/mm3 | 2685.5 ± 967 | 2808.2 ± 849 | .669 |
| Neutrophils, cells/mm3 | 3347 ± 1761 | 2481, IQR 738 | .177 |
| Eosinophils, cells/mm3 | 346 ± 292.8 | 673 ± 374.5 | .005 |
| ELR | 0.1253 ± 0.0838 | 0.2457 ± 0.1298 | .002 |
| ENR | 0.1225 ± 0.13002 | 0.3024 ± 0.22045 | .005 |
| Occasional vomiting, n (%) | 0 (0) | 2 (8.3) | .222 |
| Food impaction, n (%) | 3 (17.6) | 8 (33.3) | .264 |
| Dysphagia, n (%) | 14 (82.4) | 20 (83.3) | .934 |
| Eosinophils | ELR | ENR | |
|---|---|---|---|
| AUC (95% CI) | 0.755 (0.602–0.908) | 0.767 (0.622–0.912) | 0.782 (0.637–0.927) |
| AUC P value | .006 | .004 | .002 |
| Cut-off point | 455.05 | 0.2431 | 0.1137 |
| Sensitivity, % (95% CI) | 70.8 (48.9–87.3) | 54.1 (32.8–74.4) | 83.3 (62.6–95.2) |
| Specificity, % (95% CI) | 82.3 (56.5–96.2) | 94.1 (71.3–99.8) | 64.7 (38.3–85.7) |
| PPV, % (95% CI) | 85 (66.2–94.2) | 92.8 (65.2–98.9) | 76.9 (63–86.6) |
| NPV, % (95% CI) | 66.6 (50.8–79.4) | 59.2 (48.1–69.5) | 73.3 (51.2–87.7) |
| LR+ | 4.01 (1.39–11.5) | 9.21 (1.33–63.8) | 2.36 (1.21–4.61) |
| LR− | 0.35 (0.18–0.69) | 0.49 (0.31–0.76) | 0.26 (0.10–0.67) |
| Ppost(+), % (95% CI) | 85 (66–94) | 93 (65–99) | 77 (63–87) |
| Ppost(−), % (95% CI) | 33 (20–49) | 41 (30–52) | 27 (12–49) |
| OR (95% CI) | 11.3 (2.46–52.1) | 18.9 (2.15–166.2) | 9.16 (2.12–39.6) |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; ELR, eosinophil-lymphocyte ratio; ENR, eosinophil-neutrophil ratio; EoE, eosinophilic esophagitis; IQR, interquartile range; LR−, negative likelihood ratio; LR+, positive likelihood ratio; no EoE, no eosinophilic esophagitis (normal biopsy); NPV, negative predictive value; OR, odds ratio; Ppost(−), post-test probability of a negative result; Ppost(+), post-test probability of a positive result; PPV, positive predictive value.
(A) ROC curves for the eosinophil count, ELR and ENR for diagnosis of eosinophilic esophagitis. (B) The Fagan nomogram showed a prior probability of eosinophilic esophagitis in the study sample of 58% (prevalence) and a posterior probability after a positive result of 93% (95% CI, 65–99), as shown by the blue line. In fact, 1 in 1.1 patients with a positive ELR result (> 0.243) received a final diagnosis of EoE.
In our analysis, we found significantly higher peripheral eosinophil counts in patients with EoE, which was consistent with previous reports.5 However, the applicability of this finding is limited and, since serial endoscopies are required in the follow-up of EoE, the usefulness of noninvasive markers has been investigated in this context. A previous study found that peripheral blood absolute eosinophil count and levels of eosinophil-derived neurotoxin (EDN) and eotaxin-3 were significantly correlated to esophageal eosinophil density (eosinophil count: r = 0.56 [P = .0001]; EDN: r = 0.54 [P = .0001]; eotaxin-3: r = 0.32 [P = .04]) and were higher in patients with active EoE compared to controls (eosinophil count: 440 vs 140 eosinophils/µL [P = .05]; EDN: 50.3 vs 31.1 ng/mL [P = .01]; eotaxin-3: 37.7 vs 11.5 pg/mL [P = .01]).6 In that study, the specificity of the eosinophil count for diagnosis of EoE was 75% and the PPV 67%, with a cut-off point of 300 cells/mm3. This yield is inferior compared to the diagnostic performance observed in our study, in which the peripheral eosinophil count exhibited a specificity of 82% and a PPV of 85% for diagnosis of EoE, although the cut-off point was higher (455 eosinophils/mm3), which could partly explain these differences. In the past study, the eosinophil count performed best for diagnostic purposes, contrary to our study, in which the ELR was the marker that offered the highest accuracy with a cut-off point of 0.243, a specificity of 94% and a PPV of 92% (Fig. 1B).
Unfortunately, this is the first study to describe these cell ratios in EoE, so we were unable to compare our data. In addition, there are methodological and sample size limitations. However, although these results should be considered exploratory and require validation, in our study, the eosinophil-lymphocyte ratio exhibited a high specificity, PPV, and NPV for diagnosis of EoE. This could be useful in the diagnostic and therapeutic approach to patients with esophageal symptoms, allowing prioritization of endoscopic studies in patients with values greater than 0.243 or consideration of other etiologies in the initial differential diagnosis of children with ELRs below this threshold.
CRediT authorship contribution statementJCMA: concept, methods and statistical analysis, data handling, writing of initial draft, revision and editing. SBV, AMC, APM and MCYI: visualization, supervision, critical revision, approval and editing.
Ethical considerationsAll patients and/or legal guardians provided consent verbally and in writing to the publication of clinical data. The study was also approved by the Research Ethics Committee (No. Reg. 3318-0000206).
FundingThis research did not receive any external funding.
Data availabilityThe data that support the conclusions of this study are available through the corresponding author, JCMA, upon reasonable request.
The authors have no conflicts of interest to declare.