Recent studies show an increase in the prevalence of Haemophilus influenzae and a decrease in Streptococcus pneumoniae among the bacteria that cause acute otitis media (AOM). The objective of our study was to analyse the distribution of pathogens identified in children aged less than 14 years presenting to the emergency department with AOM and their patterns of antimicrobial resistance.
Patients and methodsSingle centre retrospective, analytical study in patients aged less than 14 years with a diagnosis of AOM in whom an ear drainage sample was collected for culture in the paediatric emergency department of a tertiary care hospital between 2013 and 2021.
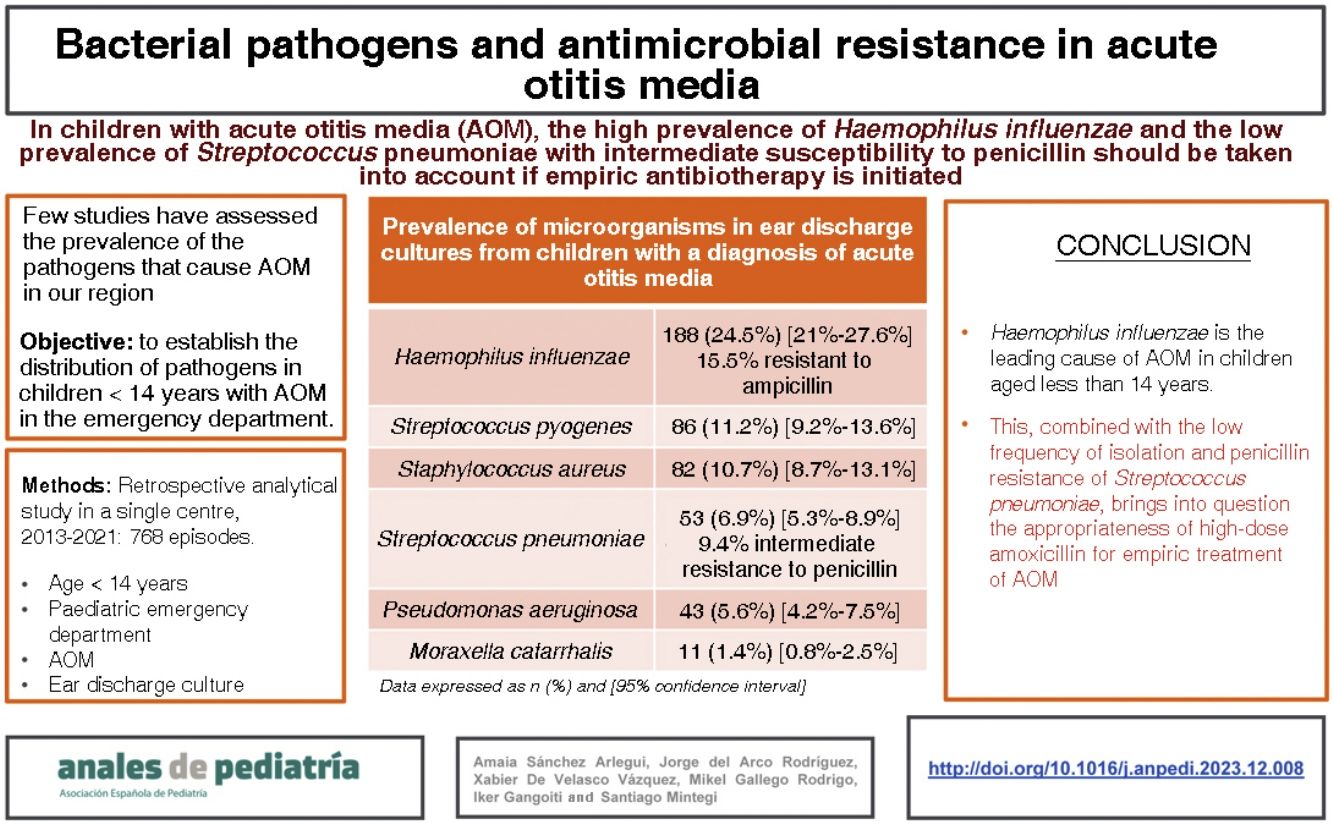
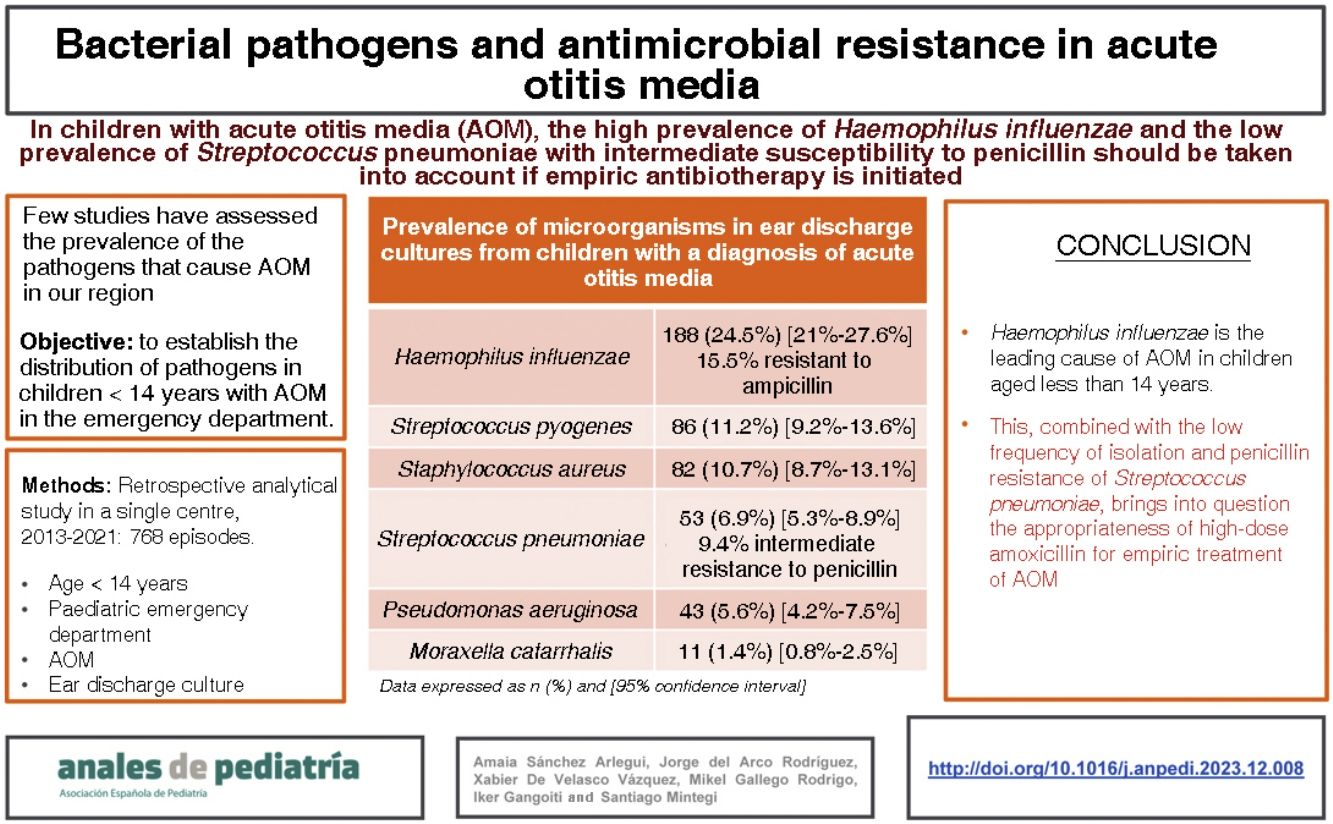
ResultsDuring the study period, there were 14 684 documented care episodes corresponding to children with a diagnosis of AOM. An ear drainage culture was performed in 768 of those episodes. The median age of the patients was 2 years, 57% were male and 70% had a previous history of AOM. The most frequently isolated pathogens were: Haemophilus influenzae (n = 188 [24.5%]; 15.5% of them resistant to ampicillin), Streptococcus pyogenes (n = 86 [11.2%]), Staphylococcus aureus (n = 82 [10.7%]), Streptococcus pneumoniae (n = 54 [6.9%]; 9.4% with intermediate resistance to penicillin), Pseudomonas aeruginosa (n = 42 [5.5%]) and Moraxella catarrhalis (n = 11 [1.4%]). No pathogen was isolated in 34.9% of cases.
ConclusionsHaemophilus influenzae is the leading cause of AOM in children aged less than 14 years. This, combined with the low frequency of isolation and penicillin resistance of Streptococcus pneumoniae, calls into question the appropriateness of high-dose amoxicillin for empiric treatment of AOM.
Estudios recientes señalan un aumento de la prevalencia de Haemophilus influenzae y una disminución de Streptococcus pneumoniae entre las bacterias causantes de otitis media aguda (OMA). El objetivo del estudio es conocer la distribución de microorganismos patógenos identificados en Urgencias en los menores de 14 años con OMA y su patrón de resistencias.
Pacientes y métodosEstudio retrospectivo, analítico y unicéntrico incluyendo pacientes menores de 14 años diagnosticados de OMA en los que se recogió un cultivo de secreción ótica en el servicio de urgencias pediátricas de un hospital terciario entre 2013 y 2021.
ResultadosDurante el periodo de estudio, se registraron 14684 episodios con diagnóstico de OMA, recogiéndose en 768 cultivo de secreción ótica. La mediana de edad fue de 2 años, el 57% varones y el 70% habían presentado al menos una OMA previa. Los patógenos más frecuentemente aislados fueron: Haemophilus influenzae 188 (24,5%; de ellos 15,5% resistentes a ampicilina), Streptococcus pyogenes 86 (11,2%), Staphylococcus aureus 82 (10,7%), Streptococcus pneumoniae 54 (6,9%, de ellos 9,4% con resistencia intermedia a penicilina), Pseudomonas aeruginosa 42 (5,5%) y Moraxella catarrhalis 11 (1,4%). En el 34.9% no se aislaron patógenos.
ConclusionesHaemophilus influenzae es la primera causa de OMA en menores de 14 años. Este hecho, junto a la baja tasa de aislamientos y resistencia a penicilina de Streptococcus pneumoniae, cuestiona la idoneidad de la amoxicilina a dosis elevadas como tratamiento antibiótico empírico de la OMA.
Acute otitis media (AOM) is one of the most frequent reasons for seeking medical care and receiving antibiotherapy in children,1,2 and is more frequent between ages 6 and 24 months.3 At present, we know that approximately 70%–92% of AOM episodes resolve spontaneously without need of antibiotherapy, so initiation of empiric antibiotherapy is recommended only in select patients based on age, whether AOM is unilateral or bilateral, the intensity of pain, the severity of fever, the presence of risk factors or the impossibility of adequate follow-up.4
The most frequent causative agents of AOM are Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis.1,5,6 The predominant pathogen is usually S pneumoniae, and the recommended empiric therapy is high-dose amoxicillin due to the high proportion of strains with intermediate penicillin resistance.
In recent years, there have been a number of relevant changes. On one hand, the restriction of the indication of treatment for select patients has been associated with a reduction in the frequency of amoxicillin resistance in S pneumoniae.7 On the other hand, following the introduction of the 7-valent and 13-valent pneumococcal conjugate vaccines (PCV7 and PCV13), the distribution of pathogens has been changing. Before the introduction of these vaccines, the S pneumoniae serotypes covered by the PCV7 accounted for 60%–70% of cases.8,9 After the introduction of the PCV13, approximately 15%–25% of cases are caused by S pneumoniae,6,10,11 while the proportion of AOM cases caused by M catarrhalis and H influenzae has been increasing, with the latter bacterium accounting for 50%–60% of cases.6 Different studies conducted outside of Spain have identified H influenzae as the most frequent aetiological agent in children.12 However, the most recent guidelines do not contemplate changing the recommended empirical therapy for children with AOM.11 Empiric therapy must target the most frequent pathogens. If the change in the aetiological agent distribution and the predominance of H influenzae were corroborated in our study, we considered that it would be necessary to adapt the recommendation for the initial empiric antibiotherapy.
Our working hypothesis was that H influenzae is the leading pathogen causing AOM in our area and that the frequency of intermediate-level penicillin resistant S pneumoniae is low.
The main objective of the study was to determine the prevalence of different bacterial pathogens and their drug resistance profile in children aged less than 14 years with a diagnosis of AOM in our autonomous community. The secondary objective was to assess the appropriateness of the empiric antibiotherapy currently recommended for children with AOM.
MethodsWe conducted a single-centre retrospective and descriptive study in patients with AOM in whom ear drainage culture was performed for diagnosis at the paediatric emergency department (PED) of a tertiary care hospital in the 2013–2021 period. This department manages approximately 55 000 episodes a year in children aged 0–14 years.
In the PED, the diagnosis of AOM, otorrhoea and mastoiditis is based on the diagnostic criteria established by the Sociedad Española de Urgencias de Pediatría (Spanish Society of Paediatric Emergency Medicine) and the International Classification of Diseases, 10th revision (ICD-10) coding system.13
We considered the following a relevant personal history:
- •
Follow-up in the Department of Otorhinolaryngology due to one of the following: recurrent AOM, serous otitis media, adenoid or tonsil enlargement, patients with ear tubes, hearing loss, cholesteatoma or any other disease involving the middle or inner ear.
- •
Current treatment that could facilitate the development of bacterial infections, such as steroids or immunosuppressant drugs.
The study included every patient aged less than 14 years given a diagnosis of AOM in whom an ear drainage sample was collected for culture (index case). In our PED, the decision to perform an ear drainage culture rests with the physicians in charge of the patient, in most cases medical residents in the speciality of paediatrics under the supervision of an emergency medicine paediatrician on site. We defined return visit as any care episode for which the same patient visited the PED again within 72 h of the initial visit.
We excluded patients with a diagnosis of AOM in whom an ear discharge culture was not performed and those with incomplete data (missing data on isolated pathogens, drug resistance profile).
The ear discharge culture was carried out by seeding the discharge on blood agar, MacConkey agar and enriched chocolate agar plates, incubated for 48 h in an aerobic atmosphere for the MacConkey agar, in anaerobic conditions for the chocolate agar and in a CO2-enriched atmosphere for the blood agar.
Antibiotic susceptibility tests were conducted an interpreted according to the current standards of the Clinical & Laboratory Standards Institute (CLSI) for each pathogen and each year.
The primary variable in the study was the ear discharge culture, while the secondary variables were the relevant elements of the personal history, demographic characteristics, clinical manifestations and findings of the physical examination, the treatment received in the PED and patient outcomes.
Statistical analysisWe have expressed qualitative variables as absolute and relative frequencies and 95% confidence intervals. We summarised continuous variables as mean and standard deviation or median and interquartile range depending on their distribution. We compared quantitative variables with the Student t test and categorical variables with the χ2 test or the Fisher exact test. We considered P values of less than .05 statistically significant.
The study was approved by the Clinical Research Ethics Committee with code E22/35.
ResultsIn the period under study, there were 441 728 documented care episodes in children aged less than 14 years, of who 14 684 received a diagnosis of AOM (3.3%). In this subset, an ear discharge sample was collected from 768 patients (5.2%). Cultures were performed more frequently in the winter months, without changes during the study period except for a decrease in years 2020 and 2021.
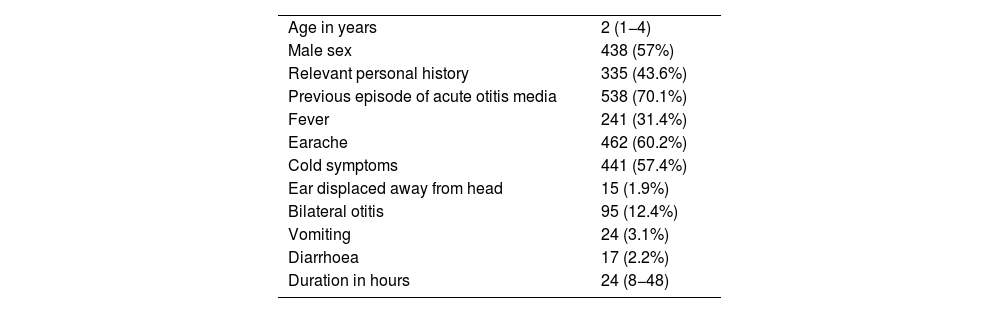
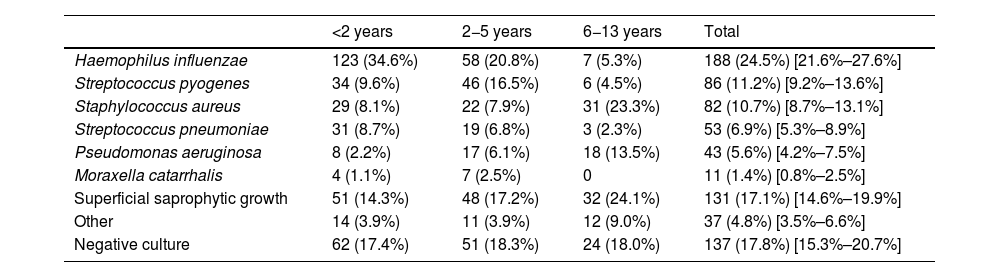
Characteristics of the patientsThe median age was 2 years, 438 patients (57%) were male, 335 (43.6%) had a relevant personal history and 538 (70.1%) had had at least one previous episode of AOM. Table 1 presents the characteristics of the 768 patients included in the study. Overall, H influenzae was the most frequently identified bacterium, present in 188 patients (24.5%) and S pneumoniae was isolated in 53 patients (6.9%), with the pathogen distribution changing significantly based on the age of the patients (Table 2).
Characteristics of the 768 patients with a diagnosis of suppurative acute otitis media in whom an ear discharge culture was performed.
| Age in years | 2 (1−4) |
| Male sex | 438 (57%) |
| Relevant personal history | 335 (43.6%) |
| Previous episode of acute otitis media | 538 (70.1%) |
| Fever | 241 (31.4%) |
| Earache | 462 (60.2%) |
| Cold symptoms | 441 (57.4%) |
| Ear displaced away from head | 15 (1.9%) |
| Bilateral otitis | 95 (12.4%) |
| Vomiting | 24 (3.1%) |
| Diarrhoea | 17 (2.2%) |
| Duration in hours | 24 (8−48) |
Data expressed as absolute frequency and percentage save for age and the duration in hours of the otitis, which are expressed as median and interquartile range.
Prevalence of microorganisms in ear discharge cultures from patients with a diagnosis of suppurative AOM by patient age.
| <2 years | 2−5 years | 6−13 years | Total | |
|---|---|---|---|---|
| Haemophilus influenzae | 123 (34.6%) | 58 (20.8%) | 7 (5.3%) | 188 (24.5%) [21.6%–27.6%] |
| Streptococcus pyogenes | 34 (9.6%) | 46 (16.5%) | 6 (4.5%) | 86 (11.2%) [9.2%–13.6%] |
| Staphylococcus aureus | 29 (8.1%) | 22 (7.9%) | 31 (23.3%) | 82 (10.7%) [8.7%–13.1%] |
| Streptococcus pneumoniae | 31 (8.7%) | 19 (6.8%) | 3 (2.3%) | 53 (6.9%) [5.3%–8.9%] |
| Pseudomonas aeruginosa | 8 (2.2%) | 17 (6.1%) | 18 (13.5%) | 43 (5.6%) [4.2%–7.5%] |
| Moraxella catarrhalis | 4 (1.1%) | 7 (2.5%) | 0 | 11 (1.4%) [0.8%–2.5%] |
| Superficial saprophytic growth | 51 (14.3%) | 48 (17.2%) | 32 (24.1%) | 131 (17.1%) [14.6%–19.9%] |
| Other | 14 (3.9%) | 11 (3.9%) | 12 (9.0%) | 37 (4.8%) [3.5%–6.6%] |
| Negative culture | 62 (17.4%) | 51 (18.3%) | 24 (18.0%) | 137 (17.8%) [15.3%–20.7%] |
Data expressed as absolute frequency and percentage with the addition of the 95% confidence interval in the total column.
P < .01.
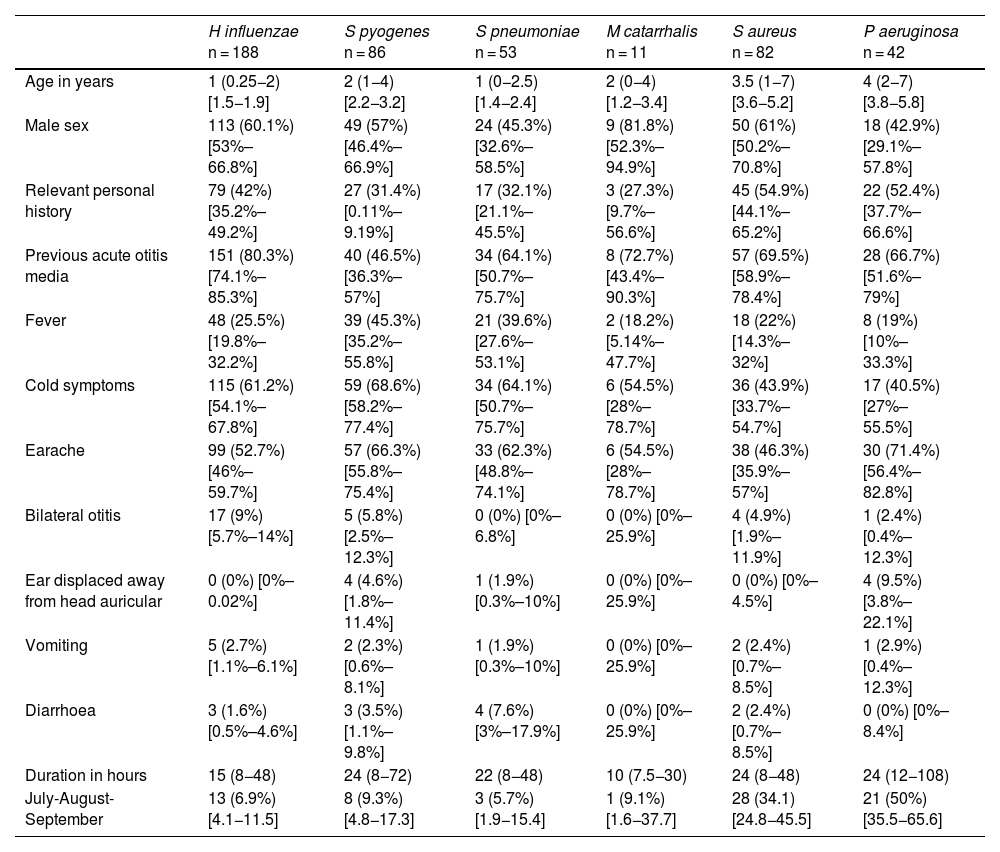
In children aged less than 5 years, H influenzae and S pyogenes were the most frequently identified bacteria. In older children, the most frequent pathogens were Staphylococcus aureus and Pseudomonas aeruginosa, usually found in children with otitis externa. The median age of the patients with isolation of S aureus (3.5 years) or P aeruginosa (4 years) was significantly higher compared to all other pathogens (1–2 years; P < .01) and these pathogens were isolated more frequently in the warm months (34.1% S aureus and 50% P aeruginosa, vs 7.4% for the rest of pathogens, P < .01). Table 3 summarises the clinical characteristics of the patients in relation to the causative agent of AOM.
Characteristics of the patients in relation to the causative agent of acute otitis media.
| H influenzae n = 188 | S pyogenes n = 86 | S pneumoniae n = 53 | M catarrhalis n = 11 | S aureus n = 82 | P aeruginosa n = 42 | |
|---|---|---|---|---|---|---|
| Age in years | 1 (0.25−2) [1.5−1.9] | 2 (1−4) [2.2−3.2] | 1 (0−2.5) [1.4−2.4] | 2 (0−4) [1.2−3.4] | 3.5 (1−7) [3.6−5.2] | 4 (2−7) [3.8−5.8] |
| Male sex | 113 (60.1%) [53%–66.8%] | 49 (57%) [46.4%–66.9%] | 24 (45.3%) [32.6%–58.5%] | 9 (81.8%) [52.3%–94.9%] | 50 (61%) [50.2%–70.8%] | 18 (42.9%) [29.1%–57.8%] |
| Relevant personal history | 79 (42%) [35.2%–49.2%] | 27 (31.4%) [0.11%–9.19%] | 17 (32.1%) [21.1%–45.5%] | 3 (27.3%) [9.7%–56.6%] | 45 (54.9%) [44.1%–65.2%] | 22 (52.4%) [37.7%–66.6%] |
| Previous acute otitis media | 151 (80.3%) [74.1%–85.3%] | 40 (46.5%) [36.3%–57%] | 34 (64.1%) [50.7%–75.7%] | 8 (72.7%) [43.4%–90.3%] | 57 (69.5%) [58.9%–78.4%] | 28 (66.7%) [51.6%–79%] |
| Fever | 48 (25.5%) [19.8%–32.2%] | 39 (45.3%) [35.2%–55.8%] | 21 (39.6%) [27.6%–53.1%] | 2 (18.2%) [5.14%–47.7%] | 18 (22%) [14.3%–32%] | 8 (19%) [10%–33.3%] |
| Cold symptoms | 115 (61.2%) [54.1%–67.8%] | 59 (68.6%) [58.2%–77.4%] | 34 (64.1%) [50.7%–75.7%] | 6 (54.5%) [28%–78.7%] | 36 (43.9%) [33.7%–54.7%] | 17 (40.5%) [27%–55.5%] |
| Earache | 99 (52.7%) [46%–59.7%] | 57 (66.3%) [55.8%–75.4%] | 33 (62.3%) [48.8%–74.1%] | 6 (54.5%) [28%–78.7%] | 38 (46.3%) [35.9%–57%] | 30 (71.4%) [56.4%–82.8%] |
| Bilateral otitis | 17 (9%) [5.7%–14%] | 5 (5.8%) [2.5%–12.3%] | 0 (0%) [0%–6.8%] | 0 (0%) [0%–25.9%] | 4 (4.9%) [1.9%–11.9%] | 1 (2.4%) [0.4%–12.3%] |
| Ear displaced away from head auricular | 0 (0%) [0%–0.02%] | 4 (4.6%) [1.8%–11.4%] | 1 (1.9%) [0.3%–10%] | 0 (0%) [0%–25.9%] | 0 (0%) [0%–4.5%] | 4 (9.5%) [3.8%–22.1%] |
| Vomiting | 5 (2.7%) [1.1%–6.1%] | 2 (2.3%) [0.6%–8.1%] | 1 (1.9%) [0.3%–10%] | 0 (0%) [0%–25.9%] | 2 (2.4%) [0.7%–8.5%] | 1 (2.9%) [0.4%–12.3%] |
| Diarrhoea | 3 (1.6%) [0.5%–4.6%] | 3 (3.5%) [1.1%–9.8%] | 4 (7.6%) [3%–17.9%] | 0 (0%) [0%–25.9%] | 2 (2.4%) [0.7%–8.5%] | 0 (0%) [0%–8.4%] |
| Duration in hours | 15 (8−48) | 24 (8−72) | 22 (8−48) | 10 (7.5−30) | 24 (8−48) | 24 (12−108) |
| July-August-September | 13 (6.9%) [4.1−11.5] | 8 (9.3%) [4.8−17.3] | 3 (5.7%) [1.9−15.4] | 1 (9.1%) [1.6−37.7] | 28 (34.1) [24.8−45.5] | 21 (50%) [35.5−65.6] |
Data expressed as absolute frequency and percentage save for age and the duration in hours of the otitis, which are expressed as median and interquartile range, in addition to the 95% confidence interval.
The distribution of bacteria did not change significantly based on the presence of a relevant history or the year under study, except for a decrease in the isolation of S pyogenes in 2020 and 2021.
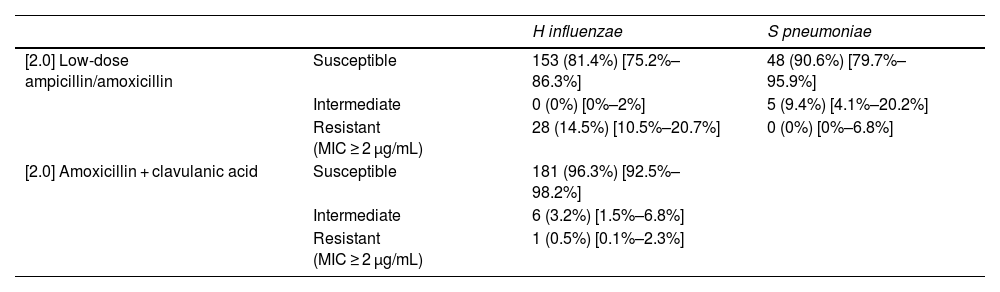
Table 4 presents the drug resistance patterns in H influenzae and S pneumoniae.
Antimicrobial drug resistance profile of H influenzae and S pneumoniae.
| H influenzae | S pneumoniae | ||
|---|---|---|---|
| [2.0] Low-dose ampicillin/amoxicillin | Susceptible | 153 (81.4%) [75.2%–86.3%] | 48 (90.6%) [79.7%–95.9%] |
| Intermediate | 0 (0%) [0%–2%] | 5 (9.4%) [4.1%–20.2%] | |
| Resistant (MIC ≥ 2 μg/mL) | 28 (14.5%) [10.5%–20.7%] | 0 (0%) [0%–6.8%] | |
| [2.0] Amoxicillin + clavulanic acid | Susceptible | 181 (96.3%) [92.5%–98.2%] | |
| Intermediate | 6 (3.2%) [1.5%–6.8%] | ||
| Resistant (MIC ≥ 2 μg/mL) | 1 (0.5%) [0.1%–2.3%] |
Data expressed as absolute frequency (percentage) [95% confidence interval].
MIC, minimum inhibitory concentration.
In all 153 patients with a diagnosis of AOM and an ear discharge culture, a strain of S pneumoniae with intermediate resistance to amoxicillin was isolated from the culture.
DiscussionThe bacterium isolated most frequently from the cultures of ear discharge samples collected from children aged less than 14 years with a diagnosis of AOM in our PED was H influenzae.
To our knowledge, this is the only study carried out recently in our area in a large sample of paediatric patients, and it corroborates the findings reported elsewhere.5,14 A systematic review that included studies conducted between 1993 and 201715 found that the bacteria isolated most frequently in children with AOM were S pneumoniae (26.1%), H influenzae (18.8%), S aureus (12.3%) and S pyogenes (11.8%), without clear changes during those years, probably on account of the limited information available regarding the pneumococcal vaccination status of the children and the limited data from recent years.15 In any case, most studies highlighted the impact of the pneumococcal conjugate vaccine and the shift in the pathogens identified in children with AOM,5,11,16 including those with severe disease.12 In our series, independently of whether the patients had nor did not have a relevant history, the most frequently identified bacterium was H influenzae, followed by S pyogenes, while S pneumoniae was isolated in slightly less than 7% of cases and isolation of M catarrhalis was rare. The only relevant change during the study period was the decrease in the frequency of isolation of S pyogenes in 2020 and 2021, a finding that must be interpreted with caution due to the impact of the protective measures established on account of the COVID-19 pandemic on respiratory diseases. These measures would also explain the decrease in the performance of cultures, given the reduction in the diagnosis of respiratory infections that occurred in those years.
Antibiotic resistance patterns must also be taken into account in the selection of empiric antibiotherapy. Approximately 15% of the H influenzae isolates were resistant to amoxicillin, which was consistent with the findings of several previous studies12 and lower compared to the 50% reported in others.17 On the other hand, none of the S pneumoniae isolates in this case series was fully resistant to penicillin (minimum inhibitory concentration [MIC] > 2), while 9.3% exhibited intermediate susceptibility. This rate of resistance is lower compared to the previous literature, in which rates of 15%–30% have been reported, due to the emergence of nonvaccine serotypes.11 Today, due to the increase in the frequency of isolates of nonvaccine serotypes15,18,19 with reduced susceptibility to amoxicillin, prescription of the standard dose is not currently recommended.11 The predominance of H influenzae in our study and the fact that S pneumoniae strains with intermediate susceptibility to amoxicillin caused approximately 1 in 150 cases of AOM brings into question the appropriateness of high-dose amoxicillin for empiric treatment of children with AOM. Our findings support the prescription of the standard dose of amoxicillin-clavulanic acid for initial treatment.11,16,17,20 However, the modification of the antibiotic must be contemplated with caution given that in current clinical practice, the real-world frequency of resolution of AOM treated with narrow spectrum versus broad-spectrum antibiotics is 96.9% versus 96.6%.21 This is probably due to the increased prevalence of organisms that are more likely to have a benign course and cause self-limited illness in addition to overdiagnosis in these patients.17 Furthermore, a shift from the prescribing of narrow-spectrum antibiotics to broad-spectrum antibiotics could significantly increase morbidity on account of the adverse effects of the latter, for instance, through increasing in the prevalence of infection by Clostridium difficile and the development of antimicrobial resistance.17 The modification of empiric antibiotherapy for AOM in children should be accompanied by the development of a robust multicentre surveillance system, clinical as well as microbiological, to monitor the impact of the change.
One possibility would be to individualise antibiotherapy, as there are certain differences in the clinical presentation of AOM based on the causative bacteria. For instance, it is known that children with a previous history of AOM are more likely to be infected by H influenzae.1 In our study, patients with AOM caused by H influenzae and S pneumoniae were younger compared to patients with AOM of all other aetiologies, while patients with AOM caused by H influenzae were more likely to have had previous episodes of AOM or have a relevant personal history, but these differences were not sufficiently large to establish a useful pattern to suspect the aetiology of AOM.
Last of all, approximately 15% of cultures led to isolation of S aureus or P aeruginosa, bacteria usually associated with otitis externa, in agreement with previous studies.12 Based on our findings, the diagnosis of AOM in a child aged more than 6 years with earache and suppuration should be made with caution, especially in the warm months, as many of these cases could actually be of otitis externa.
There are limitations to our study. On one hand, a culture was not performed in every child with a diagnosis of AOM who had ear discharge. Whether or not to order a culture was left to the judgment of the paediatrician in charge of the patient. It is possible that cultures were ordered more frequently in patients with more severe AOM, who were not healthy prior to the episode or with treatment failure. On the other hand, bacteria were identified by means of conventional culture. This may have resulted in the underestimation of the prevalence of these pathogens, as methods based on polymerase chain reaction are more accurate than culture of middle ear fluid,22 especially in the case of Moraxella catarrhalis. The study was conducted in a single centre, so its findings must be extrapolated to other settings with caution, although we believe that the results would be similar in other areas or settings with similar social and health care conditions, chiefly universal and free vaccination against pneumococcal disease. Lastly, we did not have information on the serotypes of the isolated S pneumoniae strains. Although this was not an objective of the study, it would have allowed us to determine whether the isolates in our hospital corresponded to the group of currently emerging serotypes (23B, 24F, 14 and 11A).11
Lastly, this study underscores the importance of establishing permanent surveillance systems to monitor the microbiological profiles of different infectious diseases, including antimicrobial resistance patterns.18
It is reasonable to conclude that H influenzae is now the leading cause of AOM. This fact, combined with the low frequency of isolation and penicillin resistance in S pneumoniae, challenges the appropriateness of using high-dose amoxicillin for empiric treatment of AOM. Prospective multicentre studies are required to confirm these findings.
Conflicts of interestThe authors have no conflicts of interest to declare.
Previous meetings: This study was presented at the 69 Congress of the AEP, June 1–3, 2023, and at the XXVI Annual of the Sociedad Española de Urgencias de Pediatría, May 16–18, 2022.