Early diagnosis of primary immunodeficiency such as severe combined immunodeficiency (SCID) and X-linked agammaglobulinemia (XLA) improves outcome of affected infants/children. The measurement of T-cell receptor excision circles (TRECS) and kappa-deleting recombination excision circles (KRECS) can identify neonates with severe T or B-cell lymphopaenia.
ObjectivesTo determine TRECS and KRECS levels from prospectively collected dried blood spot samples (DBS) and to correctly identify severe T and B-cell lymphopaenia.
Material and methodsDetermination of TRECS and KRECS by multiplex PCR from neonates born in two tertiary hospitals in Seville between February 2014 and May 2014. PCR cut-off levels: TRECS<15copies/μl, KRECS<10copies/μl, ACTB (β-actin)>1000copies/μl. Internal (XLA, ataxia telangiectasia) and external (SCID) controls were included.
ResultsA total of 1068 out of 1088 neonates (mean GA 39 weeks (38–40) and BW 3238g (2930–3520) were enrolled in the study. Mean (median, min/max) copies/μl, were as follows: TRECS 145 (132, 8/503), KRECS 82 (71, 7/381), and ACTB 2838 (2763, 284/7710). Twenty samples (1.87%) were insufficient. Resampling was needed in one neonate (0.09%), subsequently giving a normal result. When using lower cut-offs (TRECS<8 and KRECS<4 copies/μl), all the samples tested were normal and the internal and external controls were correctly identified.
ConclusionThis is the first prospective pilot study in Spain using TRECS/KRECS/ACTB-assay, describing the experience and applicability of this method to identify severe lymphopaenias. The ideal cut-off remains to be established in our population. Quality of sampling, storage and preparation need to be further improved.
El diagnóstico precoz de inmunodeficiencias primarias, como la inmunodeficiencia combinada grave (IDCG) y la agammaglobulinemia ligada al cromosoma X (ALX), mejora el pronóstico de los niños afectados. La medida de los T-cell receptor excision circles (TRECS) y kappa-deleting recombination excision circles (KRECS) puede identificar neonatos con linfopenias T y/o B graves.
ObjetivoCuantificar los niveles de TRECS y de KRECS de manera prospectiva a partir de muestras de sangre seca de talón para identificar de manera correcta linfopenias T y/o B.
Materiales y métodosDeterminación de TRECS y de KRECS mediante reacción en cadena de polimerasa multiplex en neonatos nacidos entre febrero y mayo del 2014. Los puntos de corte empleados fueron: TRECS < 15 copias/μl, KRECS < 10 copias/μl, ACTB (β-actina) > 1.000 copias/μl. Se incluyeron controles internos (ALX, ataxia) y externos (IDCG).
ResultadosFueron analizadas 1.068 muestras de las 1.088 recogidas (edad gestacional media: 39 semanas [38-40]; peso al nacer medio 3.238g [2.930-3.520]). La media (mediana, min/máx) copias/μl obtenidas fueron las siguientes; TRECS 145 (132, 8/503), KRECS 82 (71, 7/381) y ACTB 2838 (2763, 284/7710). Veinte muestras (1,87%) fueron insuficientes para el análisis. El re-test fue necesario en un neonato (0,09%), confirmándose resultados normales posteriormente. Empleando puntos de corte inferiores (TREC < 8 y KREC < 4 copias/μl), todas las muestras resultaron normales y se identificaron los controles internos y externos correctamente.
ConclusiónEs el primer estudio prospectivo realizado en España usando el ensayo TREC/KREC/ACTB para identificar linfopenias graves. Es necesario establecer puntos de corte adecuados para nuestra población, mejorar la toma de muestras, su almacenamiento y la preparación de las mismas.
Primary immunodeficiencies (PIDs) are a heterogeneous group of disorders comprising more than 240 different pathologies, with an approximate incidence of 1:500 live births. Congenital defects of the immune system, such as severe combined immunodeficiency (SCID) and X-linked agammaglobulinaemia (XLA) are less frequent, although they still have a considerable incidence of 1:70000 to 1:100000 live births.1,2 The vast majority of neonates with severe T- and/or B-cell lymphopaenias, including those suffering from severe PIDs, have no family history of PID and are asymptomatic in the first weeks of life.3 Due to these features, delayed diagnosis is frequent, and it is associated with considerable morbidity and mortality due to the development of severe clinical manifestations at a later point.2–4 There are highly efficacious treatment options for the most severe forms of PID, for instance, immune globulin replacement therapy for the agammaglobulinaemias; and curative treatments for SCIDs, such as haematopoietic stem cell transplantation or gene therapy.5,6 It has been demonstrated that early diagnosis followed by implementation of preventive and therapeutic measures reduces the rate of sequelae, improves the quality of life of patients, and dramatically improves survival outcomes.7,8 Likewise, early identification of patients with SCID helps prevent severe iatrogenic damage, for instance by avoiding administration of live vaccines (such as the rotavirus vaccine) currently recommended to start at 2 months of age, when most patients have yet to develop characteristic symptoms.9
The methodology needed to detect PID routinely in the neonatal period has been developed in recent years. In United States, real-time PCR has been used to identify patients with SCID by quantification of T-cell receptor excision circles (TRECs) in dried blood spot (DBS) samples.10–12 Recently, pilot studies have been conducted in Sweden and Germany to evaluate a method that allows detection of T-cell lymphopaenias (through low levels of TRECs), that are typically associated with SCID, and B-cell lymphopaenias (through low levels of kappa-deleting recombination excision circles [KRECs]) associated with other severe PIDs such as XLA or ataxia telangiectasia (AT).13–16 TRECs and KRECs are episomal DNA fragments that result from gene rearrangements that occur during T- and B-lymphocyte maturation. The excision circles do not replicate during mitosis and thus exhibit a dilution pattern that allows the quantitative estimation of cell replication.12,16 Thus, patients suffering from severe PIDs who have T- and/or B-cell lymphopaenias have very low levels of TRECs and KRECs, regardless of the molecular aetiology of the PID, which is very useful considering the genetic heterogeneity of these disorders.10,13,16
In Spain, neonates are not routinely screened for potentially lethal diseases of the immune system, despite evidence that these pathologies meet the screening criteria adapted from Wilson and Jungner, which determine the appropriateness of including specific diseases in population screenings: high morbidity and mortality, an initial latent stage, availability of an accessible and relatively non-invasive screening method, evidence of benefits of early diagnosis, availability of curative or effective treatment, and finally, a favourable cost-benefit analysis.17–19
Due to a lack of previous studies and to shortfalls in the data of national registries, we do not know the real current prevalence of severe PIDs in Spain. The results of neonatal screening for PIDs in California changed what was known about the prevalence of SCID, showing a rate of 1:70000 live births compared to previous estimates ranging from 1:100000 to 1:150000 live births.20
This paper presents the initial data of a pilot study with the purpose of: (a) finding out the magnitude of an unidentified problem and (b) providing data on the feasibility and performance of a novel methodology using samples that had been obtained previously for the established neonatal screening programme.
In a second phase of the study with a larger sample we hope to: (a) obtain reliable information on the epidemiology of T- and/or B-cell lymphopaenia in our setting; (b) learn about the aetiology of T- and/or B-cell lymphopaenia, and, lastly (c) identify neonates with lymphopaenias who could benefit from early treatment, thus improving the prognosis of patients suffering from a PID.
Materials and methodsStudy design, setting, and populationWe conducted an observational, descriptive and longitudinal study of the levels of TREC and KREC in dry blood samples obtained from neonates born at the Hospitales Universitarios Virgen del Rocío (HUVR) and the Hospital de Especialidades Virgen del Valme during the period under study (February and May, 2014). Neonates were excluded from the study if their legal guardians had not given informed consent.
Sample and data collectionHeel blood samples were dropped on Schleicher & Schuell #912 filter paper (3rd to 5th day postpartum) as part of the established routine neonatal screening process. Two 3.2mm discs were punched for each sample, and stored at 4°C until they were processed. We collected relevant demographic and clinical data for the mother and the neonates: sex, gestational age, birth weight, and pathology results from the routine neonatal screening.
Sample processing and multiplex real-time polymerase chain reaction (TRECs/KRECs/ACTB assay)
DNA was purified (DNA Purification Solution, Qiagen, Maryland, USA) and extracted (DNA Elution Solution, Qiagen, Maryland, USA) from the samples following a previously described process.13 Absolute quantification of TRECs, KRECs, and ACTB in dried blood spot samples was done following the previously described process.13 The ACTB (β-actin) copy number reflects the success of DNA extraction from DBS samples.
Quality controlsWe included internal controls (designed by plasmid cloning) and external controls that obtained through the Newborn Screening Quality Assurance Programme of the Centres for Disease Control and Prevention (CDC) in Chamblee, USA (SCID, n=2; healthy, n=2). We also tested samples from patients with a confirmed diagnosis of SCID (n=1), XLA (n=2) and AT (n=2).
Definition and interpretation of resultsBased on the lower limit of detection of the assay, of 1copy/μL for TRECs and KRECs, and on the results of previous studies, we established the following cut-off scores for an estimated 99.8% sensitivity in detecting severe T- and/or B-cell lymphopaenias: TRECs<15/μL and KRECs<10/μL with ACTB>1000/μL.13
We defined three categories based on the assay results (Table 1).
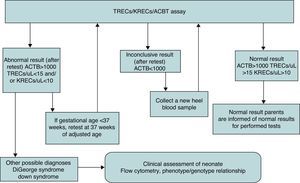
The algorithm that specified the actions to be taken after obtaining abnormal, inconclusive, or normal results is presented in Fig. 1.
In cases of abnormal or inconclusive results, the PCR assay was repeated using a new punch from the same filter paper (retest). If the results were abnormal or inconclusive in the retest, collection of another heel blood sample was requested (recall). If the results were confirmed using the new DBS sample, the legal guardians were advised to have the infant assessed by a specialist.
We also analysed the results based on cut-off points adapted to the preliminary results obtained in this study (TRECS<8/μL, KRECS<4/μL, ACTB>1000/μL). This adaptation is consistent with the experience of other groups that have used this assay (Borte et al., unpublished data).
Data analysisWe performed a descriptive analysis. Qualitative variables were expressed as absolute frequencies and percentages. Quantitative variables, depending on the normality of their distribution (assessed by means of the Kolmogorov–Smirnov tests or the Shapiro–Wilk test, n<50), were expressed as mean±standard deviation or as median and interquartile range (IQR), respectively, or by the minimum and maximum scores in the assay.
We calculated the rates of abnormal, inconclusive, and normal results for the TRECs/KRECs/ACTB assay for a 95% confidence interval. To assess the reliability of the assay, we calculated the proportion of false positive results. We analysed the causes/variables associated with or related to the presence of T- and B-cell lymphopaenias using Pearson's chi squared test or Fisher's exact test for qualitative variables; and for quantitative variables we used Student's t test for independent samples or the Mann–Whitney U test depending on the normality of their distribution.
The statistical significance level was set at P<.05. We performed the statistical analysis using the IBM software SPSS Statistics version 20.
ResultsDemographic and clinical characteristics of the sampleWe analysed 1068 blood samples collected from 1088 neonates born in the two participating centres. Of all samples, 20 (1.87%) could not be analysed because the filter paper did not have a large enough volume of blood. Most samples came from healthcare centres belonging to the service area of the HUVR (76%); 48% of the neonates included in the study were male; and 67.5% had been born at term. The mean gestational age was 39 weeks (IQR, 38–40 weeks), and the mean birth weight was 3238g (IQR, 2930–3520g). Most of the samples corresponded to newborns weighing more than 2500g (79.1%). We received some valid samples with informed consent forms that had been filled out correctly, but which were not accompanied by information about gestational age (19.9%) or birth weight (15.5%).
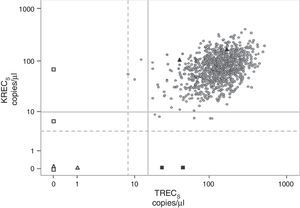
Results of the TRECs/KRECs/ACTB assayThe results of the TRECs/KRECs/ACTB assay, calculated for the two subgroups of gestational age and birth weight, are shown in Table 2 and Fig. 2. The mean and median for the three parameters (TRECs, KRECs and ACTB) were similar for the analysed subgroups.
Results of the TRECs/KRECs/ACTB assay in the general population and in relation to gestational age and birth weight.
| N | (%) | TRECS (copies/μL) | KRECS (copies/μL) | ACTB (copies/μL) | ||||||||||
| Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | |||
| GA≤37 | 135 | (12.6) | 150 | 140 | 18 | 503 | 75 | 71 | 15 | 274 | 2853 | 2744 | 352 | 5894 |
| GA>37 | 721 | (67.5) | 149 | 136 | 8 | 503 | 84 | 71 | 7 | 381 | 2017 | 2746 | 284 | 7710 |
| Unknown GA | 212 | (19.9) | 129 | 119 | 20 | 378 | 77 | 67 | 7 | 324 | 2902 | 2016 | 866 | 6528 |
| Total | 1068 | (100) | 145 | 132 | 8 | 503 | 82 | 71 | 7 | 381 | 2838 | 2763 | 284 | 7710 |
| Weight ≤2500g | 57 | (5.4) | 138 | 123 | 25 | 315 | 71 | 67 | 15 | 222 | 2920 | 2911 | 352 | 5894 |
| Weight >2500g | 845 | (79.1) | 150 | 137 | 8 | 503 | 82 | 71 | 7 | 381 | 2823 | 2746 | 502 | 7709 |
| Unknown weight | 166 | (15.5) | 123 | 116 | 18 | 415 | 79 | 66 | 11 | 324 | 2887 | 2789 | 284 | 2628 |
| Total | 1068 | (100) | 145 | 132 | 8 | 503 | 82 | 71 | 7 | 381 | 2838 | 2763 | 284 | 7710 |
GA: gestational age.
TRECs and KRECs values obtained from heel blood samples (n=1068). Circles: screened neonates; white triangles: T−B− SCID controls (CDC); black triangles: healthy controls (CDC); white squares: T−B+ and AT controls (internal); black squares: XLA controls (internal); continuous grey line: TRECs<15copies/μL and KRECs<10copies/μL; dotted grey line: TRECs<8 and KRECS<4copies/μL.
When we applied the TRECs<15/μL and KRECS<10/μL cut-off points, eight samples (0.75%) gave abnormal results. Following the established algorithm, we retested those samples taking punches from the same filter paper as before, obtaining normal results in all but one of the samples. A second filter paper needed to be prepared from a newly collected blood sample in only one neonate (0.09%) after obtaining an abnormal KRECs result (2copies/μL) in the retest. A normal result for KRECs (167copies/μL) was obtained with the second filter paper preparation.
To assess the feasibility of the assay with different cut-off points based on our sample, we used TRECs<8/μL and KRECs<4/μL. We found no abnormal results for this cut-off points in the general population. However, all the abnormal controls were identified reliably.
Prematurity and birth weightWe found no significant differences when we compared the TRECs and KRECs values of preterm newborns (none with gestational age<28 weeks) with those of full-term newborns. Likewise, we did not observe any differences between neonates with a birth weight of 2500g or lower, and neonates with birth weights greater than 2500g (Table 2).
Internal and external controlsWe analysed a total of nine controls (Table 3): one sample from a patient recently diagnosed with SCID (T−, B+ type); two samples of patients aged 3 and 17 years previously diagnosed with XLA, who were reliably identified with the assay (KRECs=0copies/μL); and two patients aged 1 and 17 years diagnosed with AT and severe lymphopaenia.
Results of internal and external controls.
| Disease | TREC (copies/μL) | KREC (copies/μL) | ACTB (copies/μL) | |
| 1 | XLA (patient) | 23 | 0 | 3229 |
| 2 | XLA (patient) | 42 | 0 | 3009 |
| 3 | AT (patient) | 0 | 7 | 805 |
| 4 | AT (patient) | 0 | 0 | 1979 |
| 5 | SCID (patient) | 0 | 78 | 932 |
| 6 | SCID (CDC) | 0 | 1 | 954 |
| 7 | SCID (CDC) | 0 | 0 | 87a |
| 8 | Healthy (CDC) | 97 | 157 | 2927 |
| 9 | Healthy (CDC) | 244 | 187 | 3770 |
AT: ataxia telangiectasia; CDC: Centers for Disease Control and Prevention; SCID: severe congenital immunodeficiency; XLA: X-linked agammaglobulinaemia.
We also analysed four samples sent by the CDC, two of which were classified as T−B− (one of them with a low ACTB copy number), and two of which had normal results.
DiscussionPatients with severe PIDs benefit from early diagnosis, as it enables prompt implementation of preventive, supportive, and therapeutic measures that improve their overall prognosis and, in the case of patients with SCID, improve survival rates through hematopoietic stem cell transplantation.5–8
Previous studies have demonstrated the reliability of the methodology based on TRECs determination in heel blood samples for identifying patients suffering from severe T-cell lymphopaenia.13,20 Considering this evidence, European experts in PID have expressed their unambiguous support for the introduction of this screening test in Europe.21 Recently, Modell et al. presented cost-benefit data that greatly favoured the implementation of neonatal screening for SCID, and proposed a model to estimate its socioeconomic impact for different populations.18 Their calculations, based on screening data from the United States, showed that the total healthcare cost for a patient with delayed diagnosis amounted to $6000000; while the cost of mass screening and the treatment and follow-up of one patient diagnosed following the screening amounted to $1385000.18 It is worth noting that the cost per neonate of the TRECs and KRECs screening in our study was €1.46, and therefore well below the cost of screening in the United States ($4.25/neonate) and the United Kingdom (£8.30/neonate), where the screening only includes the TRECs assay.
The potential of KRECs to be a marker for disorders of B-lymphocyte development in the field of routine neonatal screening has been reported in the literature.13,22 By determining TRECs and KRECs simultaneously in a single assay, its diagnostic capacity increases. Multiplex PCR (TRECs/KRECs/ACTB assay) can identify classic congenital T-lymphocyte defects (SCID), but also other immune disorders in asymptomatic newborns, such as agammaglobulinaemias, combined immunodeficiencies, and AT.14,21 The assay can also identify patients with an atypical SCID phenotype that could go undetected in assays that only determine TREC levels.23
In our pilot study, we analysed 1088 samples from 1068 neonates born in southern Spain with the aim of knowing the levels of TRECs and KRECs in this population, identifying suitable cut-off points, and assessing the feasibility of the TRECs/KRECs/ACTB assay in our setting.
Recent studies in the United Kingdom, France, and Israel, most of them retrospective, suggest that it would be beneficial to add screening for SCID to the routine neonatal screenings.22,24,25 Due to its prospective design, our study is one of the first of its kind not only in Spain, but also in Europe.
The TRECs and KRECs values observed were comparable to those previously described in other populations based on the same methodology.13 At first we used conservative cut-off values of TRECs<15/μL and KRECs<10/μL. With these cut-offs, 0.75% of the tested samples had abnormal results in our population. Other groups have reported figures between 0.08% and 4.1%, depending on the cut-off points used.13,20,21 Population-based studies with a high number of abnormal samples would be unfeasible, because of our healthcare area (with 25000 births/year) such a rate would entail having 188 samples retested every year. There are 3 possible reasons why the retest rate is this high: (a) low quality of the DBS samples taken before the analysis; (b) the learning curve for the methodology; and finally (c) conservative cut-off points. When the abnormal samples were retested, all but one gave results above the initial cut-off points (0.09%). This recall rate is considered adequate for this type of study.19
When we considered more restrictive cut-off points (TRECs<8/μL and KRECs<4/μL), we observed that all samples tested normal (not pathological). Recent data published by Gaspar et al. in the United Kingdom, and data by Borte et al. (personal communication) are similar and suggest that a specific cutoff point needs to be determined for each laboratory and population.20,26
The ultimate goal is to reduce the retest rate. To achieve this, it is important that an effort is made to obtain quality samples and to improve their quality, and that a suitable cut-off point for the Spanish population is determined. This requires ongoing communication with the associated healthcare centres. Reducing the retest rate would prevent unnecessary costs and worry in families, and in particular cases, it would preclude the need to collect a new blood sample from the newborn (recall).
Preterm neonates may get false positive results more frequently with this assay, so they need to be managed differently, as specified in Fig. 1. Our pilot study found no significant differences between the two birth weight subgroups (<2500g vs ≥2500g) and the two gestational age subgroups (<37 weeks vs ≥37 weeks), although we need to consider that our population did not include samples from extremely preterm neonates (birth weight <1000g or gestational age <28 weeks). For now, given the controversy in the literature, it seems prudent to maintain a conservative approach in neonates born preterm or requiring intensive care who have received positive results.13,22,23
Out of the four controls provided by the CDC, we correctly identified two normal samples and two abnormal samples compatible with a T−B− SCID phenotype, one of them with low ACTB copy numbers, which confirmed the diagnostic capacity of the assay.
Samples of patients previously diagnosed with XLA and AT were also analysed. Abnormal results were found for both pathologies. Patients with XLA were identified through very low copy numbers of KRECs, and both patients with AT should TRECs and KRECs values be below the established cut-off points, as had been observed in previous studies.13,15 Since these samples were not collected in the neonatal period, their value to validate the cut-off point for these pathologies is limited. Nevertheless, the fact that they gave abnormal results in the screening is worth noting, as the patients with AT (ages 1 and 17 years) had significant lymphopaenia and required prophylactic treatment against Pneumocystis jirovecii and immune globulin replacement therapy.
Last of all, we analysed a sample obtained from a patient recently diagnosed with T−B+ SCID. It is noteworthy that all patients presented clearly abnormal levels of TRECs and/or KRECs (0–1copy/μL) well below the cut-off points.
This paper is the first to present results of a prospective pilot study that used an innovative methodology on dried blood samples from Spanish newborns to identify those with severe T- and B-cell lymphopaenias. The study provides valuable information on the feasibility and diagnostic capacity of this methodology. The prospective and continuous recruitment of neonatal samples will allow us to increase the validity of the obtained data, which will help implement neonatal screening for severe PIDs at the regional and eventually national levels. The cooperation of different groups of providers in the healthcare system (nurses, neonatologists, primary care paediatricians, paediatric specialists, immunologists, and experts in metabolic disorders) is essential for its implementation to be successful. The ultimate objective is to be able to diagnose asymptomatic newborns with severe PIDs in the first weeks of life. This will allow prompt initiation of preventive and supportive measures, and even of curative treatment, improving the quality of life of these patients and their families.
Funding1. Fondo de Investigaciones Sanitarias (FIS), Instituto de Salud Carlos III (PI13/01104).
2. Fundación Pública Andaluza para la Gestión de la Investigación en Salud de Sevilla (FISEVI, “Scott Family Funding”).
3. Río Hortega grants for hiring research interns. Instituto de Salud Carlos III.
4. German Federal Ministry of Education and Research (BMBF 1315883).
AuthorshipP. Olbrich and B. de Felipe contributed equally to this work.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Olbrich P, de Felipe B, Delgado-Pecellin C, Rodero R, Rojas P, Aguayo J, et al. Primer estudio piloto en España sobre el cribado neonatal de las inmunodeficiencias primarias: TRECS y KRECS identifican linfopenias T y B graves. An Pediatr (Barc). 2014;81:310–317.