There has been a striking increase in the number of newly diagnosed cases of type 1 diabetes in children in the context of the coronavirus disease 2019 (COVID-19) pandemic.1,2
With the aim of assessing short-term pancreatic function in children with mild infection by SARS-CoV-2, we conducted a multicentre prospective study in 4 Spanish hospitals between September 2020 and June 2021. The study included 89 patients with a diagnosis of SARS-CoV-2 infection by RT-PCR or antigen testing of nasopharyngeal samples. Thirty days after the diagnosis, having obtained informed consent, we collected a fasting venous blood sample for the following tests: lipid profile, complete blood count and chemistry panel, basal insulin and C-peptide levels (chemiluminescence immunoassay) and concentration of glycated haemoglobin (HbA1c) (ion-exchange reverse-phase high performance liquid chromatography; normal range, 4%–5.7%).
The mean duration of symptoms at the time of diagnosis was 1.8 days (standard deviation [SD], 1.8). The most frequent manifestations were respiratory symptoms (51.7%) and fever (48.3%) (Table 1). Only one patient was hospitalised due to suspicion of paediatric inflammatory multisystem syndrome.
Characteristics of the patients.
| n (%) | |
|---|---|
| Sex distribution | |
| Male | 46 (51.7%) |
| Female | 43 (48.3%) |
| Age distribution (years) | |
| <1 | 7 (7.9%) |
| 1-3 | 9 (10.1%) |
| 3-6 | 12 (13.5%) |
| 6-12 | 33 (37.1%) |
| >12 | 28 (31.5%) |
| Ethnicity | |
| European/Caucasian | 78 (87.6%) |
| Latin American | 8 (9%) |
| Arab | 3 (3.4%) |
| Family history of diabetes | |
| Type 1 | 3 (3.4%) |
| Type 2 | 37 (41.6%) |
| Characteristics of SARS-CoV-2 infection | |
| Fever | 43 (48.3%) |
| Respiratory symptoms | 46 (51.7%) |
| Gastrointestinal symptoms | 20 (22.5%) |
| Changes in taste/smell | 4 (4.5%) |
| PIMS | 1 (1.1%) |
PIMS, paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2.
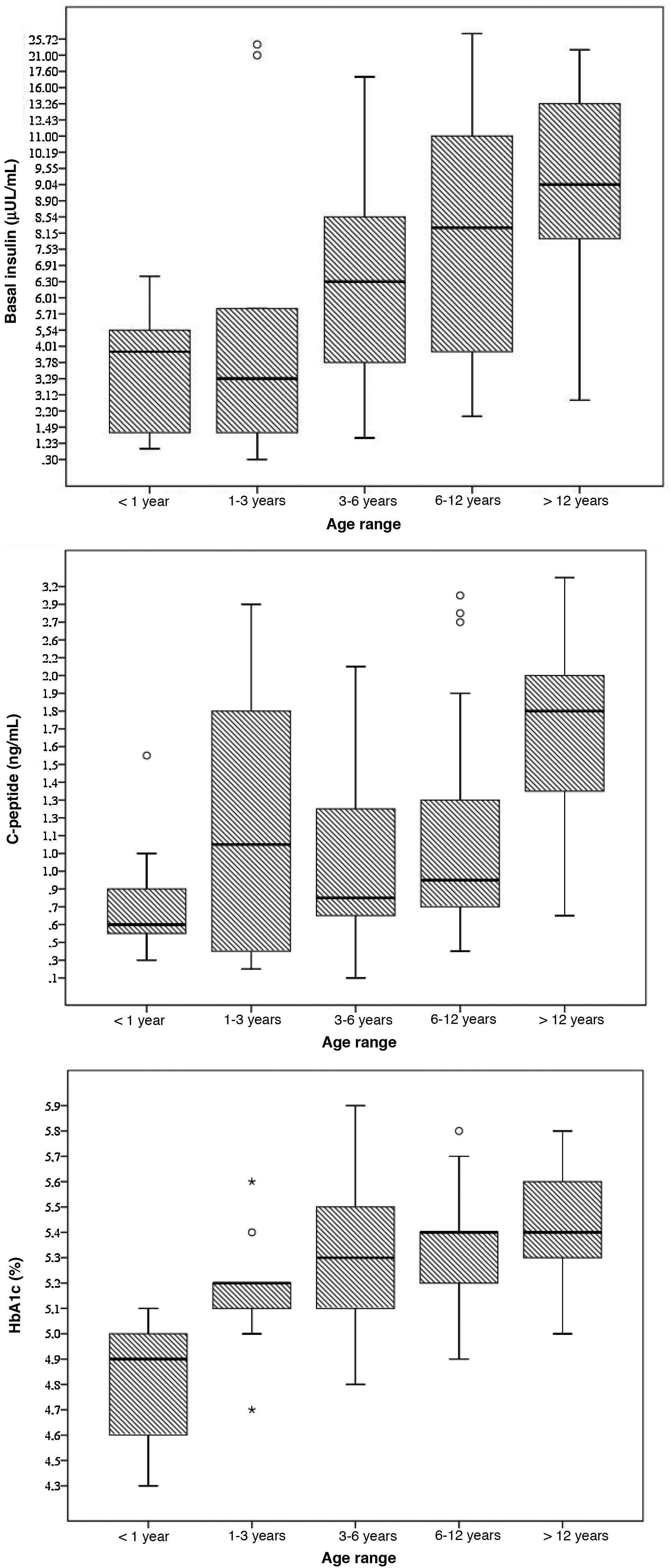
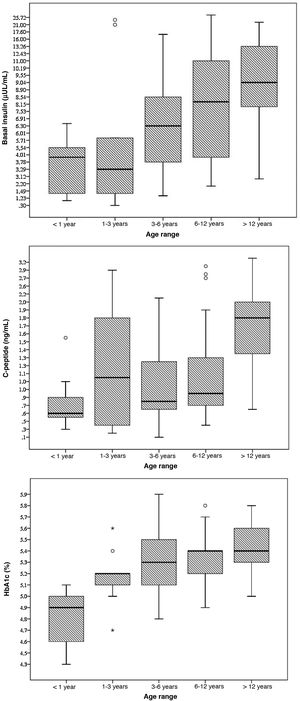
The mean concentration of HbA1c was 5.2% (significantly lower in female patients: 5.1% versus 5.3%; P = 0.006) with a statistically significant correlation between age and the HbA1c concentration (Pearson r, 0.452; P < 0.001). None of the patients had HbA1c levels of 6.5% or greater (range, 4.3%–5.9%); however, 5 patients (5.5%) had values of 5.7% or greater (80% with a body mass index [IMC] above the 90th percentile).
The mean level of C-peptide was 1.3 ng/mL (SD, 0.7), with no differences based on sex (P = 0.289). Thirty-seven percent of patients had levels of less than 1 ng/dL; in this subset, the mean HbA1c concentration was 5%. We found a statistically significant correlation between age and C-peptide levels (Pearson r, 0.456; P < 0.001).
The mean blood insulin level was 8.5 μU/mL (SD, 6), without differences based on sex (P = 0.289). We found a weak correlation between blood insulin levels and age (Pearson r, 0.392; P < 0.001) and a moderate correlation between insulin levels and BMI (Pearson r, 0.477; P < 0.001).
The mean fasting blood glucose level was 88.2 mg/dL (SD, 10.2). None of the patients met the criteria for diabetes. In 6 patients (6.6%), we found abnormal fasting glucose levels (values ranging from 100 to 125 mg/dL); 3 of these patients (50%) had a BMI above the 97th percentile, although none had dyslipidaemia and all had HbA1c concentrations below 5.7% (Fig. 1).
Based on our current knowledge on the pathophysiology of COVID-19, some authors have attributed a direct role in the development of type 1 diabetes to SARS-CoV-2.3 The virus uses the angiotensin converting enzyme 2 receptor to enter and infect host cells, and this receptor is expressed both in the lung and in the endocrine pancreas. There is ample documentation of the pancreatic damage caused by SARS-CoV-2 in adults, but when it comes to the paediatric population, this issue remains to be elucidated.
A recently published study by the Centers for Disease Control and Prevention of the United States analysed the risk of newly diagnosed diabetes (type 1, 2 or other) more than 30 days after the diagnosis of acute SARS-CoV-2 infection in patients aged less than 18 years,4 and found a significantly greater incidence of diabetes in patients with COVID-19. However, as the authors themselves noted, a percentage of these new cases of diabetes probably occurred in patients with prediabetes, a condition that is present in 1 out of 5 adolescents in the United States.5
Our findings suggest that the hypothetical pancreatic damage induced by SARS-CoV-2 would be transient and mild. None of the patients had HbA1c or fasting glucose levels meeting the criteria for diagnosis of diabetes, and while 5 patients had HbA1c concentrations of 5.7% or greater and 6 patients fasting glucose levels in the abnormal range, this was more likely related to their BMI rather than the infection by SARS-CoV-2.
Although the study was conducted in several centres, the sample was small and could not be considered representative of the general population of paediatric patients affected by COVID-19. Since similar studies have not been published before, we were unable to compare our findings with those of other researchers. We also do not know whether the cases in our sample were caused by the same variant of the virus or different variants. This could be relevant, since, as has occurred with other RNA retroviruses, as the pandemic has evolved, so has the genome of the virus and, consequently, its intrinsic characteristics related to its transmissibility or virulence.6
In conclusion, in this case series we did not find evidence of SARS-CoV-2 infection in children causing significant changes in pancreatic function or glucose metabolism, at least in the short term. Our results should be confirmed in larger population-based studies.
We thank Dr Manuel Molina Bayón and Dr María del Pilar Gutiérrez Díez.
Coordinator: Miguel Ángel Molina Gutiérrez (Paediatric Emergency Department, Hospital Universitario La Paz).
Secretary: Belén Sagastizábal Cardelús (Department of Paediatrics, Hospital Universitario de Getafe).
Members: Ana Castel-Ruíz Molinelli (Department of Paediatrics, Hospital Universitario de Getafe), Blanca Guijo Alonso (Department of Paediatrics, Hospital Universitario Ramón y Cajal), Sinziana Stanescu (Metabolic Disease Unit, Department of Paediatrics, Hospital Universitario Ramón y Cajal), Isabel González Casado (Department of Paediatric Endocrinology, Hospital Universitario La Paz), José Antonio Ruíz Domínguez (Paediatric Emergency Department, Hospital Universitario La Paz), María José Alcázar Villar (Department of Paediatrics, Hospital Universitario de Fuenlabrada).