Foetal malnutrition (FM) is the result of a loss or failure of intrauterine acquisition of the correct amount of fat and muscle mass, with short and long term implications. As the diagnosis of FM is essentially clinical, the aim of this study was to detect the incidence of FM using the Clinical Assessment of Nutritional Status (CANS) score, and compare the results with the classic anthropometric parameters.
Patients and methodsRetrospective population of term infants was studied between 2003 and 2014 (n=14,477). They were classified into adequate weight (AGA), small weight (SGA) and large weight (LGA) for gestational age newborns. The CANS score was performed on all infants enrolled in the study, and the ponderal index (PI) was calculated, considering an FM cut off value of a CANS score <25 and PI <2.2g/cm3.
ResultsUsing the CANS score, 7.6% (n=1101) of the population showed FM, 50.3% (n=538) of SGA, 76.2% (n=193) subgroupn=559) of AGA. The CANS score was <25 in 7.26% (n=1043) of newborns with PI ≥2.2g/cm3 (n=14,356), and the CANS score was >24 in 49% with PI <2.2g/cm3 (n=109).
ConclusionsIt is worthwhile identifying all newborns with FM due to the risks they may have in the short and long term.
CANS score assessment allows a better identification of nutritional status of infants than only using the curves of weight for gestational age.
La malnutrición fetal (MF) traduce una pérdida o fallo de adquisición intrauterina de la cantidad adecuada de grasa y masa muscular, asociando connotaciones pronósticas a corto y largo plazo. Siendo el diagnóstico de MF esencialmente clínico, el objetivo de este trabajo es detectar la incidencia MF mediante el Clinical Assessment of Nutritional Status score (CANS score), y comparar los resultados con los parámetros antropométricos clásicos.
Pacientes y métodosEstudio retrospectivo poblacional de recién nacidos a término entre 2003 y 2014 (n=14.477). Se clasificaron en recién nacidos de peso adecuado, pequeño y grande para la edad gestacional. Se realizó el CANS score y se calculó el índice ponderal (IP) a todos los recién nacidos incluidos, considerándose MF los puntos de corte: CANS score <25 e IP<2,2g/cm3.
ResultadosMediante el CANS score el 7,6% (n=1.101) de la población presentó MF, el 50,3% (n=538) de los recién nacidos de peso pequeño para la edad gestacional, el 76,2% (n=193) del subgrupo <p3 y el 4,67% (n=559) de los recién nacidos de peso adecuado para la edad gestacional. El CANS score fue <25 en el 7,26% (n=1.043) de los recién nacidos con IP ≥2,2g/cm3 (n=14.356), y el CANS score fue >24 en el 49% con IP<2,2g/cm3 (n=109).
ConclusionesEs conveniente identificar todos aquellos recién nacidos con MF por los riesgos que pueden presentar a corto y largo plazo. La valoración mediante CANS score permite una mejor identificación del estado nutricional de los recién nacidos que empleando únicamente las curvas de peso según la edad gestacional.
Foetal malnutrition (FM) is a clinical state characterised by intrauterine loss of or failure to acquire normal amounts of fat and muscle mass. Its assessment should be included in the evaluation of all newborns regardless of the classification of their weight for gestational age, as birth weight alone is a poor indicator of nutritional status.1
In recent years it has been observed that children with FM are more likely to have lower IQ scores, require special education, or have a neurologic disability, intellectual disability, learning disorders or seizures in late childhood compared to children without FM.1–5 Neurologic alterations may be aggravated by different circumstances during the neonatal period, and especially by hypoglycaemia and/or feeding difficulties. Furthermore, FM is associated more frequently with cardiovascular, endocrine and metabolic disorders at larger ages.6–8
Foetal malnutrition can be due to different causes, and in developed countries it is most often caused by placental insufficiency. In the past decade there has been a significant increase in pregnancies in older mothers, following assisted reproductive technologies, and in women with chronic or systemic diseases, which are associated with a higher incidence of preterm birth and low birth weight. Thus, there is renewed interest in assessing for FM as accurately as possible. Traditionally, assessment of the foetal nutritional status in the newborn has relied on various anthropometric parameters, such as the head circumference to length ratio (HC/L), the arm circumference to head circumference ratio (AC/HC) and Rohrer's ponderal index (PI), the latter of which is the most commonly used.1,6,9,10
There is also a clinical score that does not appear often in the literature, the Clinical Assessment of Nutritional Status (CANS) validated by Metcoff in 1994. It is easy to learn and quick to administer, and consists of evaluating nine superficial clinical signs that differentiate between newborns with adequate nutrition and malnutrition.11
The aim of our study was to determine the incidence of FM in term newborns in our hospital by means of the CANS score, and whether weight for gestational age and calculation of the PI suffice for the assessment of FM.
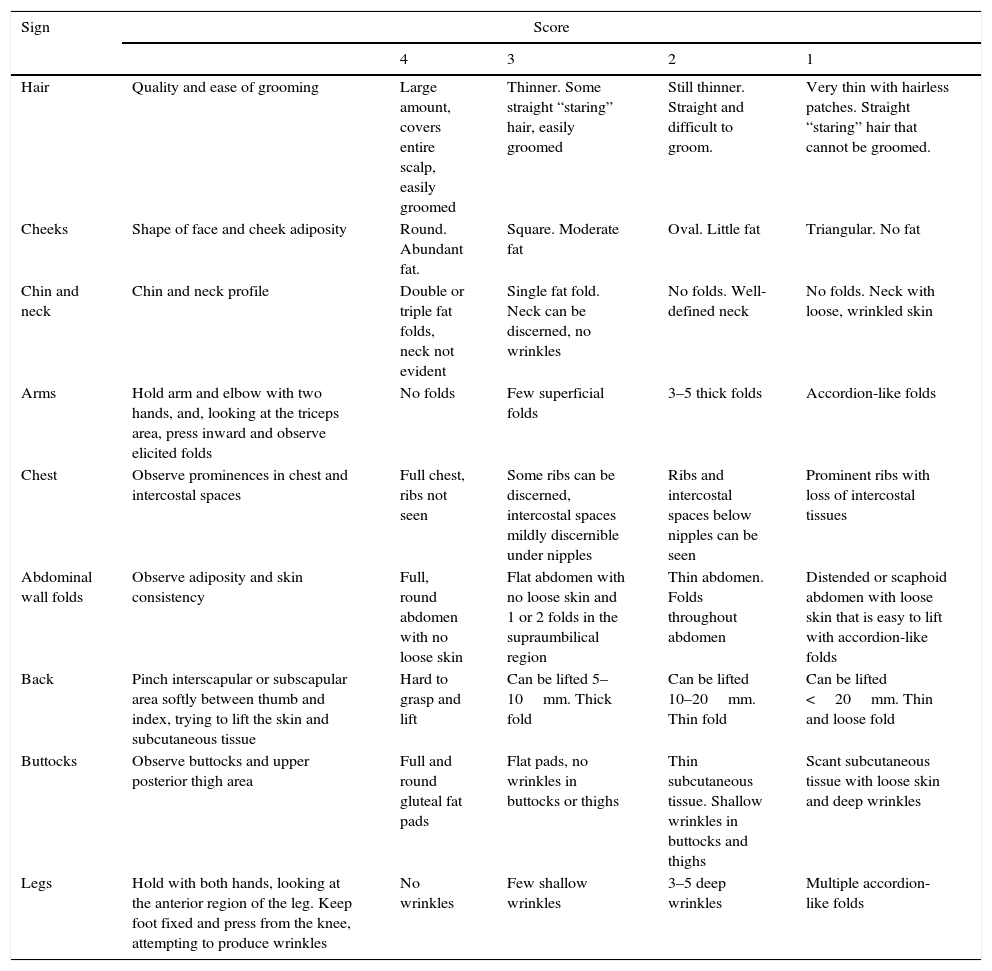
Patients and methodsWe conducted a retrospective study in a population of term neonates (born at 37–41 weeks’ gestation), with no exclusions, between March 2003 and March 2014. Gestational age was determined based on the first day of the last menstrual period and/or the first trimester ultrasound, and measured in completed weeks. We collected the following data: birth weight, length and head circumference within 24h postbirth. Weight was measured placing the unclothed newborn on a digital SECA® scale with a measuring range of 0.1–15kg and an accuracy of ±5g; the length was determined by measurement of the crown-heel length with a rigid Maciá® stadiometer with a 0–80cm range and an accuracy of ±0.5cm; and head circumference (occipitofrontal) with a flexible measuring tape accurate to 0.5cm. We entered these anthropometrical measurements to the Neosoft® database, which uses the DGPS curves of the Generalitat de Catalunya 200812 as the reference to classify newborns into adequate for gestational age (AGA, 10th–90th percentile), large for gestational age (LGA, >90th percentile) or small for gestational age (SGA, <10th percentile), and identified a subgroup of SGA with weights below the 3rd percentile. We calculated the PI for all included newborns at the end of the study applying the formula published by Rohrer in 1921: PI=weight (g)×100/length3 (cm). The PI increases as gestation advances and plateaus when the pregnancy reaches term, with a PI of 2.3g/cm3 corresponding to the 10th percentile and a PI of 2.2g/cm3 to the 3rd percentile. We considered a PI <2.2g/cm3 a sign of malnutrition,1,4,13 and also used this index to classify SGA newborns as SGA type I (symmetrical, PI>2.2g/cm3) and SGA type II (asymmetrical, PI<2.2g/cm3). We performed the evaluation by means of the CANS score within the first 24h postbirth in all neonates, assessing the nine clinical signs described by Metcoff (Table 1). Each sign is scored on a range of 1–4, and the final total score ranges between 9 and 36, with FM defined as a total score of less than 25.
Description of CANS score.
| Sign | Score | ||||
|---|---|---|---|---|---|
| 4 | 3 | 2 | 1 | ||
| Hair | Quality and ease of grooming | Large amount, covers entire scalp, easily groomed | Thinner. Some straight “staring” hair, easily groomed | Still thinner. Straight and difficult to groom. | Very thin with hairless patches. Straight “staring” hair that cannot be groomed. |
| Cheeks | Shape of face and cheek adiposity | Round. Abundant fat. | Square. Moderate fat | Oval. Little fat | Triangular. No fat |
| Chin and neck | Chin and neck profile | Double or triple fat folds, neck not evident | Single fat fold. Neck can be discerned, no wrinkles | No folds. Well-defined neck | No folds. Neck with loose, wrinkled skin |
| Arms | Hold arm and elbow with two hands, and, looking at the triceps area, press inward and observe elicited folds | No folds | Few superficial folds | 3–5 thick folds | Accordion-like folds |
| Chest | Observe prominences in chest and intercostal spaces | Full chest, ribs not seen | Some ribs can be discerned, intercostal spaces mildly discernible under nipples | Ribs and intercostal spaces below nipples can be seen | Prominent ribs with loss of intercostal tissues |
| Abdominal wall folds | Observe adiposity and skin consistency | Full, round abdomen with no loose skin | Flat abdomen with no loose skin and 1 or 2 folds in the supraumbilical region | Thin abdomen. Folds throughout abdomen | Distended or scaphoid abdomen with loose skin that is easy to lift with accordion-like folds |
| Back | Pinch interscapular or subscapular area softly between thumb and index, trying to lift the skin and subcutaneous tissue | Hard to grasp and lift | Can be lifted 5–10mm. Thick fold | Can be lifted 10–20mm. Thin fold | Can be lifted <20mm. Thin and loose fold |
| Buttocks | Observe buttocks and upper posterior thigh area | Full and round gluteal fat pads | Flat pads, no wrinkles in buttocks or thighs | Thin subcutaneous tissue. Shallow wrinkles in buttocks and thighs | Scant subcutaneous tissue with loose skin and deep wrinkles |
| Legs | Hold with both hands, looking at the anterior region of the leg. Keep foot fixed and press from the knee, attempting to produce wrinkles | No wrinkles | Few shallow wrinkles | 3–5 deep wrinkles | Multiple accordion-like folds |
During the period under study, the CANS score was performed by six paediatricians that had been previously trained in its use. The interrater variability during the training period, which included the first 400 assessments, was of ±1 point.
We analysed the data with the statistical software R Core Team 2013. We used 2×2 contingency tables to assess the association between the different parameters under study. We calculated Pearson's correlation coefficient and linear regression to assess the correlation between the CANS score and the weight for gestational age.
ResultsA total of 14,477 newborns were included for the period under study, and we found an incidence of FM of 7.6% using the CANS score, in which 1101 newborns had a score of less than 25.
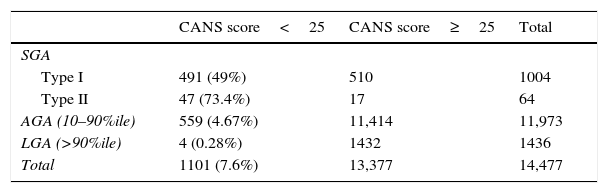
Our analysis by weight percentile showed a CANS score of less than 25 in 76.2% (193) of newborns with weights below the 3rd percentile, 42.3% (345) of those with weights between the 3rd and 10th percentiles, 4.67% (559) of AGA newborns and 0.28% (4) of LGA newborns. The analysis by PI in SGA newborns found a CANS score of less than 25 in 49% (491) of SGA type I and 73% (47) of SGA type II (Table 2).
Distribution of clinical foetal malnutrition based on the CANS score by category of weight for gestational age.
| CANS score<25 | CANS score≥25 | Total | |
|---|---|---|---|
| SGA | |||
| Type I | 491 (49%) | 510 | 1004 |
| Type II | 47 (73.4%) | 17 | 64 |
| AGA (10–90%ile) | 559 (4.67%) | 11,414 | 11,973 |
| LGA (>90%ile) | 4 (0.28%) | 1432 | 1436 |
| Total | 1101 (7.6%) | 13,377 | 14,477 |
GA, adequate for gestational age; LGA, large for gestational age; SGA, small for gestational age; %ile, percentile.
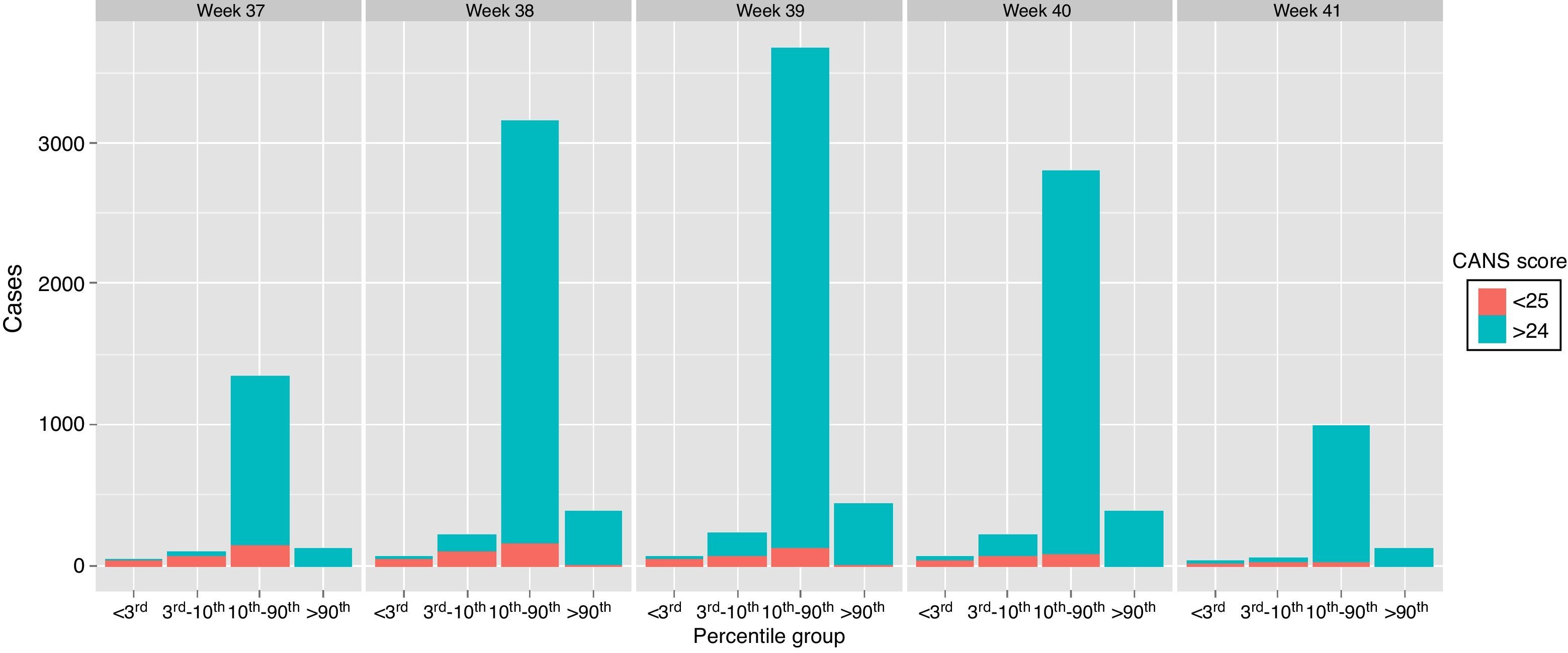
Figure 1 shows the incidence of FM as determined by the CANS score by weeks of gestation and weight percentile, with scores below 25 found in all weight and gestational age groups.
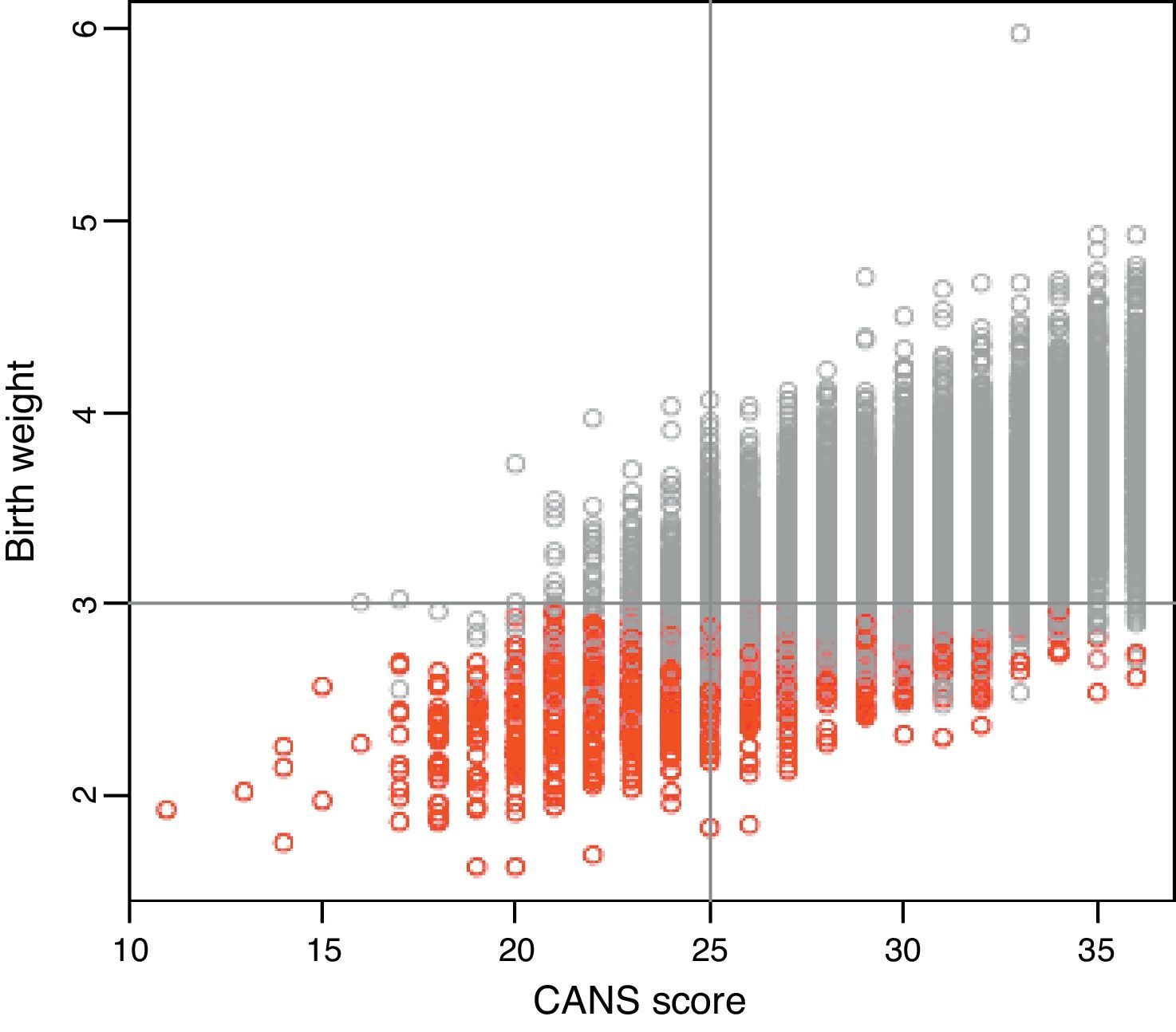
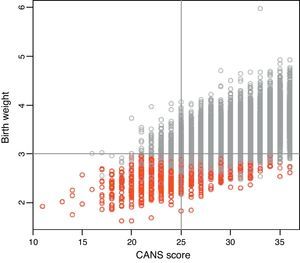
Figure 2 shows a positive correlation between CANS score and birth weight (r, 0.625; P<0.0001), and it is worth noting that most SGA cases correspond to CANS scores of less than 25, while the proportion of SGA cases decreases with increasing CANS scores. There were newborns classified as SGA based on weight for gestational age curves that did not meet the clinical criteria for FM and, conversely, newborns classified as AGA or LGA with clinical manifestations of FM.
We found 109 newborns with PIs <2.2g/cm3, of which 49% had CANS scores below 24. Of all newborns with PIs ≥2.2g/cm3 (14,356) 7.26% (1043) had CANS scores below 25. We found a positive correlation between the CANS score and the PI (r, 0.4172; P<0.0001).
DiscussionFoetal malnutrition, which was first described by Scott and Usher in 1966, is defined as failure to acquire adequate amounts of fat and muscle mass during intrauterine development.1,4,7,13 Different terms are used to try to classify or identify nutritional deficiencies in the foetus: SGA, intrauterine growth restriction and placental insufficiency, although none of them is synonymous with FM.2,4,14,15
It has been demonstrated that FM is associated with a higher risk of perinatal morbidity and mortality secondary to foetal distress, meconium aspiration, asphyxia, neonatal hypoglycaemia or hyperviscosity syndrome.7,16 In the middle and long term it is also associated with a higher risk, compared to the general population, of neurologic or mental disorders (cognitive and learning disorders) and endocrine, metabolic and cardiovascular complications,3,6–8,14,17–19 so it is important that newborns with FM are identified early to provide adequate management and monitoring.
Intrauterine growth is one of the most important indicators of foetal wellbeing. Foetuses that suffer malnutrition may adapt to it by altering their hormone production or the sensitivity of tissues to different hormones, with insulin playing a key role in the regulation of foetal growth. The adaptations undergone by the foetus may lead to permanent changes in structure, organ function and different metabolic pathways, a phenomenon known as “fetal programming”.2,8,18,20 Newborns that have gone through this adaptive process in utero can benefit from postnatal intervention to prevent to the extent possible the complications that may arise in the immediate neonatal period, as this adaptation is maintained in the first weeks of life.2 At birth, these babies show diminished subcutaneous fat and underlying muscles, and malnutrition is most evident in the arms, legs, elbows, knees and interscapular region.9
Anthropometry has been and continues to be extensively used for assessment of intrauterine growth because of its effectiveness and low cost. When weight, length and head circumference and the indices obtained by combining them are associated with gestational age, they are even more informative. The most commonly used parameters are the head circumference to length ratio (HC/L), the arm circumference to head circumference ratio (AC/HC) and Rohrer's ponderal index (W/L3×100), the latter of which is the most widely used in neonatology, as it is more sensitive than birth weight for the identification of neonatal risk of morbidity associated with intrauterine growth abnormalities and is not affected by sex or ethnicity.10 However, there is considerable doubt that these indices suffice to identify all children with malnutrition since, to give an example, the PI does not correlate strongly to measures of subcutaneous fat.4,14 The CANS clinical score validated by Metcoff does make an indirect assessment of subcutaneous fat and can detect FM in all newborns, while none of the other anthropometric measurements that assess the thickness of subcutaneous tissue perform better.13 It is a systemic assessment, easy to learn and to implement, and while adding it to the routine evaluation of the newborn does lengthen its duration, this increase is not significant after the initial learning period. It could be very useful for screening for FM and provide information for early intervention. It would be particularly useful in maternity units with fewer specialised staff or in rural areas with poor obstetric follow up where the gestational age of newborns may not be determined with certainty, which need an easy and valid method to determine the nutritional status of the newborn.
The classification of weight for gestational age is commonly used in most hospitals or maternity units to determine which interventions will be performed. However, this classification does not take into account the foetal growth potential (the weight the newborn would have achieved if foetal nutrition had been adequate), and newborns without FM may be classified as SGA, while others with clinical signs of FM may be classified as AGA.9 Our study showed that 4.67% of newborns classified as AGA presented clinical signs of FM, which means that the exclusive use of curves of weight for gestational age can lead to underdiagnosis of a considerable proportion of newborns with FM that could probably benefit in the short and long term from interventions addressing this problem. Conversely, we observed that among the newborns classified as SGA type I that accounted for 94% of SGA, 51% did not show signs of FM, and in these patients it may be more beneficial to determine the reason why they were born smaller than the rest of the population, that is, whether they were constitutionally small with no suspicion of associated morbidity or, if necessary, ruling out a malformation or a genetic, toxic or infectious aetiology.
Many studies have been published regarding the short- and long-term health risks in newborns with intrauterine growth restriction and/or signs of FM. On the other hand, there is very little information on how to best identify these newborns in the immediate neonatal period, and most studies rely solely on the classification of weight for gestational age. There are few studies that, like our own, classify newborns based on the CANS score and analyse the validity of this instrument for detecting FM, and most of them have been conducted in developing countries.1,4,9,20 While the incidence of FM is higher in these countries (17–28%), we cannot overlook that it can reach up to 10.9% in developed countries.11 Our study found an incidence of FM of 7.6% based on the CANS score, and a percentage of SGA of 7.37%; while these percentages are similar, they do not overlap, since we found different percentages of clinical FM based on the type of SGA (49% in SGA type I and 73% in SGA type II). A more interesting and relevant finding that must be underscored is that of all newborns with clinical signs of FM (7.6%), 50.8% belonged to the AGA group. The results of this study highlight the need to use the CANS score to assess FM in all newborns in developed countries, too, as discharging these babies, which occurs increasingly early following birth to facilitate attachment, would not be beneficial.
When it came to the detection of FM by means of the PI, which we studied due to the widespread use of this parameter, our results showed that while it is a better tool to detect FM than the classification of weight for gestational age, 7.26% of the assessed newborns that had PIs ≥2.2g/cm3 had clinical signs of FM, which means that a considerable percentage of the population would be underdiagnosed or misdiagnosed.
ConclusionsWeight for gestational age as a sole indicator does not suffice to identify FM in all newborns. Since FM is considered a clinical state, the CANS score could be useful for identifying FM in newborns. However, it would be preferable if it could be fully validated based on biochemical parameters. For the time being, the combination of PI and CANS score allows a better assessment of nutritional status.
Conflict of interestsThe authors have no conflict of interests to declare
Please cite this article as: Martínez-Nadal S, Demestre X, Raspall F, Vila C, Álvarez J, Sala P. Valoración clínica del estado nutricional fetal al nacer mediante el clinical assessment of nutritional status score. An Pediatr (Barc). 2016;84:218–223.