Inflammatory myofibroblastic tumours (IMTs) are infrequent lesions of intermediate malignant potential that develop in the first decades of life.1,2 They have been described to appear in multiple sites, with the lung being most common,1 followed by the abdomen and, more rarely, the brain, orbit, soft tissues and genitourinary tract.2,3 Their aetiology is unknown, although some predisposing factors have been described, such as a history of surgical intervention, repeated traumatic injury, chronic infection or radiation therapy.2,3
The clinical picture varies depending on the involved region. Local symptoms may develop due to a mass effect, such as cough, haemoptysis, chest pain or atelectasis in case of lung involvement or abdominal pain or melena in case of abdominal involvement.1 Up to 40% of IMTs are asymptomatic and detected by chance in imaging tests.1 They are frequently associated with anaemia, thrombocytosis, elevation of the erythrocyte sedimentation rate (ESR) and hypergammaglobulinaemia, especially in abdominal tumours.1 The findings of imaging are nonspecific, usually with visualization of a well-defined, irregular solid lesion, with calcifications in 25% of cases.2 In contrast-enhanced imaging, IMTs may exhibit delayed-phase heterogeneous enhancement.2 Diagnosis requires ruling out other possible tumours, abscesses and chronic infections (by Aspergillus or mycobacteria).1 The diagnosis is confirmed by histology, with visualization of spindle-shaped myofibroblasts and variable amounts of inflammatory cells (eosinophils, plasma cells and lymphocytes),2,3 and the tumours are usually positive for vimentin, actin, CD34 and CD117.2,3 Anaplastic lymphoma kinase (ALK) reactivity is observed in 50% of cases, more frequently in younger patients, but not in cases of distant metastasis, which are usually ALK-negative.4
The standard of care is complete resection of the tumour, which is curative in most cases.2 However, close monitoring is required, as in some cases the tumour can recur or become malignant and require adjuvant treatment such as chemotherapy, radiation therapy or immunosuppressive drugs.1 In the case of ALK-positive tumours, there is a specific therapeutic option: ALK inhibitors.5,6 These drugs play an important role, as they can be used in cases in which resection would be complicated as neoadjuvant therapy or in case of local or metastatic recurrence, thereby achieving an increase in overall survival.5 Crizotinib was one of the first such drugs to be used, but there have been reports of poor initial response or development of resistance after a few months of treatment, which stimulated the development of second-generation ALK inhibitors (ceritinib, alectinib) that have shown promising results.6
With the aim of understanding the characteristics of the cases managed in a tertiary care hospital, we conducted a retrospective descriptive study in patients aged less than 16 years managed between 2005 and 2020, and identified 5 cases of IMT. We analysed the following variables: age at diagnosis, sex, presentation at onset, tumour location, diagnostic tests performed, treatment and outcome (Table 1).
Series of cases of inflammatory myofibroblastic tumour reviewed in our hospital.
| Sex | Age (years) | Site | Presentation | ALK | Actin | Vimentin | First-line treatment | Recurrence | Followup (years) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IHC | FISH | ||||||||||
| 1 | F | 1 | Lung | Weight faltering and anaemia | NP | NP | NP | NP | Surgery | No | 13 |
| 2 | M | 2 | Liver | Fever and hepatomegaly | NP | NP | + | NP | Surgery | No | 6 |
| 4 | M | 5 | Lung | Fever and constitutional symptoms | + | NP | + | + | Surgery | No | 8 |
| 3 | M | 12 | Ribcage | Pain in ribcage and constitutional symptoms | − | + | + | − | Surgery | Yes | Died |
| 5 | F | 8 | Paravesical | Abdominal pain and dysuria | + | + | − | + | Surgery | No | 3 |
F, female; FISH, fluorescent in situ hybridization; IHC, immunohistochemistry; M, male; NP, not performed.
We included a total of 5 cases, corresponding to 3 boys and 2 girls. The mean age at diagnosis was 5.6 years (range, 1.8–12.2 years). Of all tumours, 2 were located in the lung, 2 in the abdomen and 1 in bone. In this case series, the most frequent clinical manifestations at onset were associated with the mass effect (pain in the ribcage, abdominal pain and seizures), although all patients also had constitutional symptoms. One patient received an initial diagnosis of pulmonary tuberculosis and another patient a diagnosis of liver abscess. The salient findings of laboratory tests were thrombocytosis (in 4 patients), anaemia (in 3) and elevation of C-reactive protein elevation (in 3), ESR (in 2) and lactate dehydrogenase (in 2).
The initial diagnosis (Fig. 1) was confirmed in all cases by the pathological examination of a biopsy sample, supplementing the histological examination with immunohistochemistry and in situ fluorescence in the most recent cases (3/3 ALK +; 3/4 actin +; 2/3 vimentin +). All patients underwent complete resection of the tumour, with favourable outcomes in 4 out of the 5 observed in the followup (median duration, 7 years). One patient experienced malignant transformation of the tumour (inflammatory fibrosarcoma) and early relapse after surgery, requiring adjuvant therapy with ceritinib, which achieved a quick initial response, but ultimately with progression in a few months resulting in death.
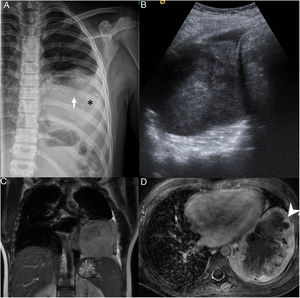
(A) Radiograph of the ribcage showing infiltration and partial destruction of the posterior shaft of the left 8th rib (white arrow) associated with a large intrathoracic, extrapulmonary soft-tissue mass with faint amorphous calcifications (*) and mild pleural effusion. (B) Ultrasound image of the heterogeneous mass in the thorax. (C) T2-weighted magnetic resonance imaging (MRI), coronal plane and (D) intravenous contrast-enhanced, fat-saturated T1-weighted MRI, transversal plane, showing a lobulated mass in contact with the mediastinal, pericardial, axillary and diaphragmatic pleura with peripheral enhancement (arrow point) and necrosis in the central area.
Inflammatory myofibroblastic tumours have an intermediate malignant potential. Their presentation varies depending on their anatomical location and systemic inflammatory manifestations. The management is based on tumour location, feasibility of surgical resection, the course of disease and ALK expression. Treatment with ALK inhibitors has shown promising results. The findings in our series are similar to those reported in the previous literature. We contribute information on a tumour located in the ribcage, a site for which the available data is scarce.
Please cite this article as: Martínez Navarro G, Pérez Chamorro M, Veiga Canuto D, Juan Ribelles A, Fernández Navarro JM. Casuística de tumor miofibroblástico inflamatorio en centro terciario. An Pediatr (Barc). 2021;95:364–366.