Drug-induced liver injury due to chemotherapy is an important cause of morbidity in cancer patients, although its clinical manifestations are poorly understood.
ObjectiveThe objective of the present study was to determine the characteristics (forms of presentation, severity, and type of injury) of hepatotoxicity due to chemotherapy in children treated for cancer.
Patients and methodA total of 22 oncological patients were included in the study, after ruling out other causes of increased transaminases (infectious, metabolic, autoimmune, or hereditary), according to the CIOMS causality scale, it is concluded that it was a possible, probable or definite episode of hepatic injury by drugs.
ResultsAll children had more than one episode of hepatotoxicity, and a total of 98 episodes are analysed. Methotrexate was the most commonly implicated drug. The histological pattern of predominant damage was hepatocellular. Only 2 episodes were classified as serious.
ConclusionsIdiosyncratic hepatotoxicity due to chemotherapy is frequent, with a tendency to relapse with re-exposure. Although it does not usually have important consequences, the high frequency makes it advisable to establish standardised safety algorithms with very strict monitoring of liver enzymes during high periods of risk in chemotherapy.
La lesión hepática inducida por fármacos debida a quimioterapia es una causa importante de morbilidad en enfermos oncológicos aunque sus manifestaciones clínicas son poco conocidas.
ObjetivoEl objetivo del presente estudio fue determinar las características (formas de presentación, gravedad y tipo de lesión) de la hepatotoxicidad por quimioterapia en niños tratados por cáncer.
Pacientes y métodoSe incluyó en el estudio a un total de 22 enfermos oncológico en los que, tras descartar otras causas de aumento de transaminasas (infecciosa, metabólica, autoinmune o hereditaria), se concluye, según la escala de causalidad CIOMS, que se trata de un episodio posible, probable o definido de lesión hepática por fármacos.
ResultadosTodos los niños tuvieron más de un episodio de hepatotoxicidad, en total se analizan 98 episodios. Metotrexato fue el fármaco implicado con mayor frecuencia. El patrón histológico de daño predominante fue hepatocelular. Solo 2 episodios fueron clasificados de graves.
ConclusionesLa hepatotoxicidad idiosincrásica por quimioterapia es frecuente, la tendencia es a la recidiva con la reexposición y, aunque no suele tener consecuencias importantes, la elevada frecuencia hace aconsejable establecer algoritmos de seguridad estandarizados con controles muy estrictos de enzimas hepáticas durante los períodos de alto riesgo de quimioterapia.
Chemotherapy-induced liver injury (CILI) is a significant cause of morbidity in oncological patients. Most hepatotoxicity secondary to chemotherapy is idiosyncratic and there is no particular clinical or histological feature differentiating chemotherapy from other drugs that damage the liver.1 The main mechanisms underlying CILI involve the production of reactive metabolites generated by phase I oxidation reactions, immunologic damage or changes in mitochondrial function. Chemotherapy-induced liver injury may also be modified by underlying liver disease or whether the tumour is in the liver itself.1
While the benefits of chemotherapy (remission of cancer) are high, so are the risks that the oncologist and patient must be willing to accept.
At any rate, the hepatotoxicity secondary to drugs used for cancer treatment is still poorly assessed due to a relative underestimation of their clinical impact and the difficulty involved in differential diagnosis.2
Acute lymphocytic leukaemia (ALL) is a malignancy that accounts for 25% of all malignant tumours in children.3,4 Approximately 80% of affected patients can be cured, but treatment failure and the cytotoxic effects of treatment continue to be serious problems in clinical practice.4,5 As a result, the focus of research is slowly shifting from an emphasis in increasing survival to reducing the toxicity caused by chemotherapy.6 At present, oral 6-mercaptopurine and weekly doses of methotrexate constitute the cornerstone of maintenance therapy for ALL.3 Despite their substantial benefits, these drugs are associated with high levels of hepatotoxicity and myelosuppression, which in many cases limit their use.7 In fact, the toxicity secondary to chemotherapy is a common cause of morbidity and mortality in children with ALL and of sequelae in the medium and long terms. These adverse events often result from direct toxicity to healthy tissues due to the low specificity of these drugs and become more frequent as treatment is intensified.
In this context, it is obvious that any factor capable of altering the pharmacokinetics or pharmacodynamics of chemotherapy agents may be critical to the development of severe adverse events in patients with ALL.
The aim of our study was to determine the characteristics (presentation, severity and type of injury) of hepatotoxicity secondary to chemotherapy in children treated for cancer.
Study sample and protocolOur sample consists of the children included in the Spanish registry of toxic liver disease in the paediatric population. This registry was originally founded in Granada in 2008. Through 3 grant-funded projects, it has gathered data on 193 suspected cases of hepatotoxicity in children. The aim of this registry is the prospective surveillance of liver injury secondary to the use of drugs and herbal products in the paediatric population by the creation of a multicentre and multidisciplinary network for the study of CILI in paediatrics.
The operational structure of the register, the methods of data collection and the definition of cases have been described in detail in a previous publication.8
The criteria used to define CILI for the purpose of including patients in the study were establishment of a temporal relationship between exposure to the drug and the onset of hepatitis combined with the presence of any of the following conditions: elevation of alanine aminotransferase (ALT) levels to at least 5 times the upper limit of normal (ULN); elevation of alkaline phosphatase (ALP) to at least twice the ULN, elevation of ALT to at least 3 times the ULN with simultaneous elevation of bilirubin levels to at least twice the ULN. The pattern of liver injury can be evaluated by calculating the R value, where R=(ALT/ULN)/(ALP/ULN), with R greater or equal to 5 reflecting a hepatocellular pattern of injury, R less than 5 but greater than 2 a mixed pattern of injury and R less than or equal to 2 reflecting a cholestatic pattern of injury.9
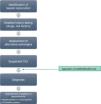
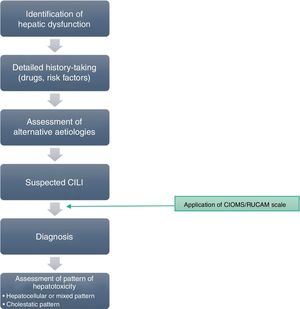
If any of the warning signs detailed above was detected, the case was notified through a structured protocol to rule out possible alternative causes. For all reported cases, a detailed medical history was taken to assess previous biliary or hepatic disease and to collect information of risk factors for liver disease. Prior to the diagnosis of hepatotoxicity, patients underwent serologic tests for detection of markers of acute viral hepatitis, measurement of serum ceruloplasmin levels and a panel for detection of antibodies associated with autoimmune liver disease. Fig. 1 presents the evaluation protocol.
After ruling out infectious, metabolic, autoimmune and inherited disorders, we included those cases that met the clinical criteria and where the causality assessment concluded the involvement of the drug was possible, probable or definite based on application of the CIOMS/RUCAM scale,9–11 which evaluates criteria related to chronicity, the course of disease, risk factors, the existing evidence on the hepatotoxicity of the drug, exclusion of other possible causes and the response to readministration of the drug (Table 1).
CIOMS/RUCAM scale.
| Hepatocellular type | Cholestatic or mixed type | Assessment | |
|---|---|---|---|
| Time to onset | |||
| Incompatible | Reaction occurred before starting the drug or more than 10 days after stopping the drug (except for slowly metabolised drugs) | Reaction occurred before starting the drug or more than 30 days after stopping the drug (except for slowly metabolised drugs) | Unrelated |
| Unknown | When information is not available to calculate the time of onset | Insufficiently documented | |
| Initial treatment | Subsequent treatment | Initial treatment | Subsequent treatment | Score | |
|---|---|---|---|---|---|
| From the beginning of the drug | |||||
| Suggestive | 5–90 days | 1–15 days | 5–90 days | 1–90 days | +2 |
| Compatible | <5 days or >90 days | >25 days | <5 days or >90 days | >90 days | +1 |
| From cessation of the drug | |||||
| Compatible | <15 days | <15 days | <30 days | <30 days | +1 |
| Course of reaction | Difference between the peak of ALT (SGOT) and upper limit of normal values | Difference between the peak of ALP (or TBIL)) and upper limit of normal values | |
|---|---|---|---|
| After cessation of drug | |||
| Highly suggestive | Decrease >50% in 8 days | Not applicable | +3 |
| Suggestive | Decrease >50% in 30 days | Decrease >50% in 180 days | +2 |
| Compatible | Not applicable | Decrease <50% in 180 days | +1 |
| Inconclusive | No information or decrease >50% after the 30th day | Persistence or increase or no information | 0 |
| Against the role of drug | Decrease <50% after the 30th day or recurrent increase | No situation. Not applicable | −2 |
| If the drug is continued | |||
| Inconclusive | All situations | All situations | 0 |
| Risk factors | Ethanol | Ethanol or pregnancy | |
|---|---|---|---|
| Presence | +1 | ||
| Absence | 0 | ||
| Age >50 years | +1 | ||
| Age <50 years | 0 |
| Concomitant treatment | |
|---|---|
| • None or no information or concomitant drug incompatible with time of onset | 0 |
| • Concomitant drug with compatible or suggestive time to onset | −1 |
| • Concomitant drug known as hepatotoxin and with compatible or suggestive time to onset | −2 |
| • Concomitant drug with evidence for its role in this case (positive rechallenge or validated test) | −3 |
| Search for non-drug causes | ||
|---|---|---|
| Group I (6 causes) | All causes (groups I and II) reasonably ruled out | +2 |
| Recent viral infection with HAV (IgM anti-HAV antibody) o HBV (IgM anti-HAV antibody) or HCV (anti-HCV antibody and negative test for A and B), biliary obstruction (ultrasonography), alcoholism (AST/ALT >2), recent history of acute hypotension (particularly in case of underlying heart disease) | The 6 causes of group I reasonably ruled out | +1 |
| Group II | 4 or 5 causes of group I reasonably ruled out | 0 |
| Complications of underlying diseases | Fewer than 4 causes of group I reasonably ruled out | −2 |
| Clinical or biological context suggesting infection by CMV, EBV or herpes virus | Non-drug cause highly probable | −3 |
| Previous information on hepatotoxicity of the drug | |
|---|---|
| • Reaction labelled in the product characteristics | +2 |
| • Reaction published but unlabelled | +1 |
| • Unknown reaction | 0 |
| Response to readministration | |||
|---|---|---|---|
| • Positive | Doubling of ALT with the drug alone | Doubling of ALP (or TBIL) with the drug alone. | +3 |
| • Compatible | Doubling of ALT with the drugs already given at the time of the first reaction | Doubling of ALP (or TBIL) with the drugs already given at the time of the first reaction | +1 |
| • Negative | Increase of ALT but by less than N in the same conditions as for the first administration | Increase of ALP (or TBIL but by less than N in the same conditions as for the first administration | −2 |
| • Not done or not interpretable | Other situations | Other situations | 0 |
We conducted the study in accordance to the most recent version of the Declaration of Helsinki. The study was approved by the ethics committee of our province. We obtained the written informed consent of the parents or legal guardians of all patients for inclusion in the study.
ResultsThe study included a total of 22 patients, 14 male and 8 female. The mean age of the sample was 5.45 years, with a range of 1–13 years. Table 2 presents the main epidemiological and laboratory characteristics of the patients.
Epidemiological and laboratory characteristics of the patients.
| Case | Sex | Age | Underlying disease | Number of episodes | Phase of treatment | Cytostatic agent | Cumulative dose(PEG-ASP in IU, rest of drugs in mg) | Mean ALT (U/L) | Severity | International normalized ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 5 | ALL | 5 | Consolidation, maintenance | MTX | 12935 | 331 | Not severe | Normal |
| 2 | Male | 4 | ALL | 3 | Consolidation, maintenance | MTX | 9680 | 758.3 | 1 severe, 3 not severe | 1.8 |
| 3 | Male | 3 | ALL | 2 | Maintenance | 6-MP | 2790 | 347 | Not severe | Normal |
| 4 | Male | 5 | ALL | 3 | Maintenance | MTX+6-MP | 19100+14332 | 324.7 | Not severe | Normal |
| 5 | Female | 3 | ALL | 4 | Induction, maintenance | 6-MP | 4120 | 353.5 | Not severe | Normal |
| 6 | Female | 4 | ALL | 4 | Consolidation, maintenance | MTX+6-MP | 7801+3612 | 581.7 | Not severe | Normal |
| 7 | Male | 7 | ALL | 10 | High-risk blocks, maintenance | PEG-ASP, thioguanine, 6-MP | 7801583.410695 | 523.3 | Not severe | Normal |
| 8 | Female | 5 | ALL | 3 | Maintenance | MTX+6-MP | 15700+11100 | 732.3 | Not severe | Normal |
| 9 | Male | 4 | ALL | 2 | Maintenance | MTX+6-MP | 13361+7812 | 447.5 | Not severe | Normal |
| 10 | Female | 5 | ALL | 4 | Induction, reinduction, maintenance | 6-MP | 2420 | 442.25 | Not severe | Normal |
| 11 | Male | 1 | ALL | 3 | Maintenance | MTX+6-MP | 11100+8940 | 340 | Not severe | Normal |
| 12 | Male | 5 | ALL | 5 | Consolidation, intensification, maintenance | MTX | 13470 | 597.6 | Not severe | Normal |
| 13 | Female | 3 | ALL | 6 | Induction, maintenance | 6-MP | 5100 | 829.3 | Not severe | Normal |
| 14 | Female | 12 | ALL | 4 | Induction, intensification, maintenance | MTX | 24860 | 333.25 | Not severe | Normal |
| 15 | Male | 5 | ALL | 7 | Induction, intensification, maintenance | ASPMTX+6-MP | 83200, 8471+18085 | 353.7 | Not severe | Normal |
| 16 | Female | 9 | ALL | 5 | Reinduction, maintenance | Thioguanine, MTX | 462, 22344 | 392.8 | Not severe | Normal |
| 17 | Male | 6 | ALL | 4 | Consolidation, intensification, maintenance | MTX+6-MP | 11370+3641 | 491.25 | 1 severe, 3 not severe | 1.7 |
| 18 | Male | 5 | ALL | 6 | Induction, maintenance | 6-MP | 13512 | 617.8 | Not severe | Normal |
| 19 | Male | 3 | Lymphoblastic lymphoma | 7 | Maintenance | MTX+6-MP | 13200+12948 | 282.7 | Not severe | Normal |
| 20 | Male | 5 | ALL | 4 | Induction, maintenance | 6-MP | 6123 | 354 | Not severe | Normal |
| 21 | Male | 13 | Lymphoblastic lymphoma | 4 | Reinduction, maintenance | Thioguanine, MTX+6-MP | 1344, 34578+16454 | 426.5 | Not severe | Normal |
| 22 | Male | 8 | Histiocytosis | 3 | Maintenance | MTX+6-MP | 281+4752 | 436.3 | Not severe | Normal |
ALL: acute lymphoblastic leukaemia; ASP: asparaginase; MTX: methotrexate; PEG-ASP: pegylated asparaginase; 6-MP: 6-mercaptopurine.
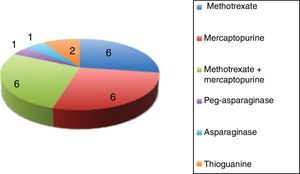
All the patients had more than 1 episode of CILI. In total, we analysed 98 episodes of hepatotoxicity. The drug involved most frequently in episodes of CILI was methotrexate, which was involved in 94 of the 98 episodes: methotrexate alone in 62 (63.3%) and in association with mercaptopurine in 32 (32.6%). The mean cumulative dose of methotrexate at the time of onset of CILI was 11086.2mg (range, 70–34578mg).
Most of the children (n=19; 86.4%) were receiving treatment for ALL, 2 (9%) for lymphoma and 1 (4.5%) for histiocytosis.
The treatment protocol applied to the children was the SHOP 2005 protocol in 9 of the patients with ALL (47.3% of children with ALL) and the SEHOP-Pethema 2014 protocol in 10 of the patients with ALL (52.63%). The treatment protocol used in patients with lymphoma was the Euro-LB02 protocol.
Fig. 2 presents the drugs or drug combinations that caused CILI in our sample.
As for the phase of treatment when the first episode of CILI developed, 6 patients had it during induction, 6 during consolidation, 7 during maintenance, 2 during IIb reinduction and 1 in treatment for high-risk block R-2.
The predominant pattern of injury based on the R value calculated with the results of the analysis of the first blood sample after detection of CILI was the hepatocellular pattern (n=90), followed by the mixed pattern (n=8); there were no episodes of cholestatic CILI.
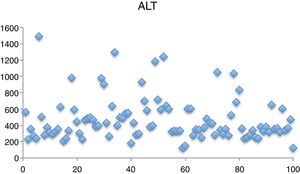
The mean levels identified in liver function tests were aspartate aminotransferase (AST), 208.72L (43–929); ALT, 471.8U/L (123–1488) (Fig. 3); ALP, 240.72U/L (63–402); gamma-glutamyl transpeptidase, 54.75U/L (10–220) and bilirubin, 1.04mg/dL (0.3–3.47).
Based on the assessment of causality by means of the CIOMS/RUCAM scale,12 we classified the diagnosis of CILI as possible in 1 case (4.5%), probable in 4 (18.2%) and highly probable or definite in 17 (77.3%).9
When it came to severity, only 2 of the episodes manifested with coagulopathy and were therefore considered serious.13
Eighteen patients underwent an ultrasound examination, which revealed a pattern of steatosis in 3 and hepatomegaly in 2, with no abnormal findings in the rest.
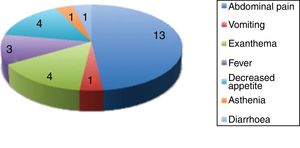
Fig. 4 presents the clinical manifestations associated with the elevation of liver enzymes.
The approach taken in relation to the treatment that caused the episode of CILI consisted in continuing treatment with a reduced dose in 42 of the episodes of hepatotoxicity (42.8%) and in temporary discontinuation of treatment with a dose reduction in the following cycle in 46 cases (46.9%). In 7 instances (71%) the clinician chose to discontinue treatment temporarily and postpone the following cycle, in which the dose was reduced. In 1 case, treatment was withdrawn completely. In 3 episodes the clinician chose watchful waiting and did not modify the dosage or schedule of treatment.
DiscussionAlthough hepatotoxicity secondary to drugs used in cancer treatment is a well-known phenomenon, to date no other studies in the literature have analysed episodes of CILI in paediatric oncological patients with a standardised approach.
The selection of an antineoplastic regimen for an oncological patient is based chiefly on the availability of effective medicines and then on the balancing of the potential toxicities associated with treatment against the clinical condition of the patient and any existing comorbidities.12 Although hypertransaminasaemia is frequently found in these patients, it is often difficult to determine its aetiology. Immunosuppression, paraneoplastic manifestations, infectious diseases, metastases and polypharmacy can muddle the assessment. Standardised protocols like the one used in the current study are needed to better understand the hepatotoxicity secondary to chemotherapy.
The two major categories of CILI are intrinsic/dose-dependent and idiosyncratic/dose-independent. The first refers to drugs that may cause liver injury in humans or animal models in a predictable manner when administered at high enough doses. The fact that transaminase elevation recurred in 100% of the patients in our sample despite reduction of the dose in 95.9% suggests that episodes of CILI are idiosyncratic and therefore dose-independent.
Since CILI remains a diagnosis of exclusion, it is one of the most challenging disorders facing paediatric oncologists. The aetiology of CILI appears to be multifactorial.14 Drug-related risk factors play a significant role, and in our study, methotrexate was the drug involved in 95.9% of documented hepatotoxicity episodes. As for patient-related factors, the most significant identified in our study was a previous history of CILI, as the reintroduction of the drug was associated with new episodes of hepatotoxicity in every instance. There is also evidence on the role of environmental factors in hepatotoxicity in oncological patients: the metabolic characteristics of the patient (such as obesity), the type of diet, the consumption of alcohol, coffee and tobacco, polypharmacy, immunological status and nutritional status.14–16 We were unable to analyse such environmental factors in our study due to the small number of patients in our sample. Large-scale multicentre studies are required to establish the importance of environmental factors.
After a positive drug challenge, patients that benefit from a drug that is critical in the management of their disease or even life-saving, as is the case of oncological patients, may be considered for a drug rechallenge,17 as occurred in our sample. Recent prospective studies that have analysed hundreds of cases of drug rechallenge have led to a current definition of new positive exposure to the drug as ALT elevation by 3–5 times the ULN or greater, which generally develops much quicker than in the initial episode of CILI.12,14,15 Drug rechallenge may be appropriate for critical medicines when safer alternatives are not available and the benefits to the patients exceed the risks.16
From a nosographic perspective, there is a tendency to attempt to differentiate patterns of liver injury and associate specific drugs with each pattern. However, it is not always possible to establish such associations. The clinical pattern of liver injury may vary based on the interaction of drug-related factors (dose, bioavailability and duration of treatment) and patient-related factors (age, sex and drug absorption).18 In our study, we were unable to detect differences in the pattern of CILI in association with different drugs, as methotrexate was involved in most episodes of hepatotoxicity (95.9%), either alone (63.3%) or in association with mercaptopurine (32.6%). Furthermore, 91.8% of adverse event were hepatotoxicity reactions. The mixed pattern of injury was rare (8.2%) and there were no cases of cholestatic injury.
We used the traditional markers of liver injury (ALT, ALP and bilirubin); but we ought to highlight that in children with cancer, patterns of liver injury may be determined by pathological processes and underlying mechanisms previously described in the literature, such as apoptosis, necrosis and necroptosis, inflammation, oxidative stress and the activation of the immune system.18 We need to identify additional markers of CILI allowing the early detection of hepatotoxicity and that may guide a more accurate diagnosis in the context of the different factors that may be at play in oncological patients.
A recent study reported that a previous history of hepatic steatosis may affect the susceptibility to CILI and the pattern of injury.19 We do not know the clinical relevance of this factor in the paediatric population, in which hepatic steatosis is the leading cause of hypertransaminasaemia.
The diagnosis of CILI is based on the exclusion of other possible aetiologies. In oncological patients treated with antineoplastic drugs, the identification of severe CILI is particularly challenging due to the complexities of polypharmacy in cancer treatment and because the mechanisms by which antineoplastic drugs may cause liver injury in susceptible individuals are not yet fully understood.20–22 For this reason, there were paediatric oncological patients with transaminase elevation that could not be included in the register because the CIOMS/RUCAM scale did not categorise them as cases of possible CILI due to a lower probability on account of the underlying disease and the stringent case definition criteria.
Although standardised criteria have been established to define the pattern of liver injury, dosage modifications are frequently empirical and based on the judgment of the clinician. Thus, clinicians need to have an exhaustive understanding of hepatotoxic manifestations of the most widely used chemotherapy agents.20 In our study, the decision made most frequently by the clinician in charge was to reduce the dose of the drug (95.9% of cases), either continuing with the established treatment schedule or postponing the following cycle. At present, there are no established guidelines for determining the most suitable approach once an episode of CILI is detected. The oncologists involved in the management of the children included in this study chose different approaches in response to the detection of hypertransaminasaemia (chiefly watchful waiting, maintenance of current dose with postponement of following cycle, dose reduction or postponement of following cycle); thus, standardised criteria were not applied to the management of this situation.
The 100% positive rechallenge rate calls for the controlled and prudent reintroduction of any drugs that have caused a first episode of CILI. Two episodes of hepatotoxicity were severe, which highlights the need to establish standardised safety algorithms with strict monitoring of liver enzymes during periods of high risk. We did not consider the possibility of definite cessation of the agents that caused episodes of CILI because they were critical drugs, so that the objective benefits to the patients exceeded the risks.
FundingThis study was funded by the Instituto de Salud Carlos III and the European Regional Development Fund (ERDF) (project numbers: PI12/00378, SAS-PI-0239/2012, AC-0073-2013). The CIBERehd is funded by the Instituto de Salud Carlos III. The funding sources did not participate in the design of the study protocol, the collection, analysis and interpretation of data, the writing of the manuscript or the decision to submit the paper for publication.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Urrutia-Maldonado E, Abril-Molina A, Alés-Palmer M, Gómez-Luque JM, Muñoz de Rueda P, Ocete-Hita E. Lesión hepática inducida por quimioterapia en niños. An Pediatr (Barc). 2019;91:256–263.