Clostridium difficile is the leading cause of nosocomial and antibiotic-associated diarrhoea in adults, and its incidence has substantially risen over the last few years. The prevalence of this infection in children is difficult to assess due to the high rates of colonisation in this setting.
Materials and methodsA one-year retrospective study was conducted on children under 15 years admitted to hospital with acute diarrhoea. Epidemiological, clinical, laboratory findings and outcome of children with Clostridium difficile infection (CDI) were compared to other causes of diarrhoea. Risk factors for CDI were identified by multivariate analysis.
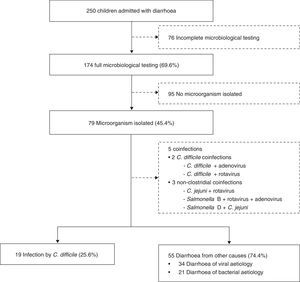
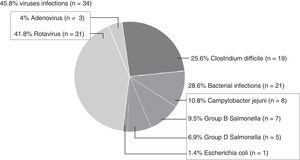
ResultsTwo hundred and fifty children with acute diarrhoea were identified. A microbiological pathogen was identified in 79 (45.4%) of 174 patients who underwent complete testing: 19 CDI (25.6%, 13 of which were enterotoxin-producing), 21 other bacteria (28.6%), and 34 viruses (45.8%; rotavirus n=31; adenovirus n=3). The estimated incidence of CDI was 3 cases/1000 admissions, with 68.4% of them occurring in children younger than 2 years. Overall, 15.8% were community-acquired. Compared to other causes of diarrhoea, CDI was associated with comorbidity (P<.0001), recent contact with the health-care system (P<.0001) or intensive care unit stay (P=.003) and exposure to antibiotics in the previous month (P<.0001). The clinical course of children with CDI was less symptomatic. There were no clinical differences between Clostridium difficile toxin-producers and non-toxin producers. Comorbidity was identified as the main risk factor associated with CDI (OR 40.02, 95% CI 6.84–232.32; P<.0001).
ConclusionsThe isolation of Clostridium difficile is common in hospitalized children with diarrhoea in our setting. CDI is more frequent in children with comorbidity and recent contact with the health-care system, presenting a mostly oligosymptomatic clinical course. Further studies are needed to understand the epidemiology of this infection in paediatrics, especially the percentage of asymptomatic carriers.
Clostridium difficile es la principal causa de diarrea nosocomial en adultos, y su incidencia está aumentado en los últimos años. Es difícil determinar su impacto en niños debido a las altas tasas de colonización.
Material y métodosEstudio retrospectivo en menores de 15 años ingresados con diarrea a lo largo de un año. Se estudiaron las características epidemiológicas, clínicas, analíticas y la evolución de los niños con infección por Clostridium difficile (ICD) en comparación con otros aislamientos. Los factores predictores de ICD fueron determinados mediante análisis multivariante.
ResultadosSe identificaron 250 niños con diarrea, realizándose estudio microbiológico completo en 174. En 79 (45,4%) se llegó al diagnóstico: 25,6% ICD (n=19; 13 enterotoxigénicos); 28,6% otras bacterias (n=21) y 45,8% virus (n=34; rotavirus n=31; adenovirus n=3). Un 68,4% fueron menores de 2 años, y un 15,8% fueron adquiridos en la comunidad. En comparación con otras causas de diarrea, la ICD se asoció a comorbilidad (p<0,0001), contacto reciente con el sistema sanitario (p<0,0001), estancia en UCI (p=0,003) y exposición reciente a antibióticos (p<0,0001). Los pacientes con ICD cursaron de forma oligosintomática. No hubo diferencias clínicas entre las ICD productoras o no de toxina, siendo la comorbilidad el principal asociado con la ICD (OR 40,02; IC 95% 6,84–232,32; p<0,0001).
ConclusionesEl aislamiento de Clostridium difficile es frecuente en niños hospitalizados con diarrea en nuestro medio. La ICD resultó más frecuente en niños pequeños con comorbilidad y contacto reciente con el sistema sanitario, presentado, en su mayoría, un curso clínico oligosintomático. Se necesitan más estudios para conocer la epidemiología de esta infección en niños.
Clostridium difficile (CD) is the main cause of nosocomial diarrhoea in adults, and is associated with significant morbidity and mortality and increased healthcare costs. Its significance in children has yet to be properly established due to the high rates of asymptomatic colonisation in infants and children less than 2 years of age, which may be as high as 70%.1,2 Although symptomatic infections in children usually involve a mild and self-limiting course of diarrhoea, some high-risk groups, such as patients with cancer or certain gastrointestinal diseases, may develop severe forms of pseudomembranous colitis.3,4
In recent years, there has been a global increase in the prevalence, morbidity and mortality of infection by CD (CDI), partly due to the emergence of the hypervirulent strain BI/NAP1/027,5 of which the first case in the Spanish population was recently described.6,7 In the paediatric population, the epidemiology of CDI has also shifted in the past decade, with an increase in the number of both hospital- and community-acquired cases.8 However, there are no studies analysing its prevalence and determining factors in Spanish children. Thus, we designed a retrospective study of hospitalised patients in order to describe the epidemiology, clinical characteristics and outcome of children in whom CD is isolated, comparing them to those of children admitted for diarrhoea caused by other pathogens.
Materials and methodsWe conducted a retrospective study of a cohort of children admitted to the Hospital General Universitario Gregorio Marañón with diarrhoea between September 2010 and October 2011. We identified the patients searching for the ICD-10 codes for diarrhoea, gastroenteritis, colitis and infection by Clostridium difficile. We performed a descriptive analysis and then divided the cohort in two groups: (1) children in whom CD was isolated, and (2) children in whom the isolated microorganism was a virus or a bacterium other than CD. We did a descriptive analysis of both groups and compared their epidemiological and clinical characteristics, laboratory findings and outcomes.
Inclusion criteriaOur analysis included patients less than 15 years of age admitted with diarrhoea that underwent full microbiological stool testing: stool culture, rotavirus and adenovirus antigen tests, and CD culture or toxin detection test9 (in our hospital, a CD culture is routinely performed for all samples processed for stool culture).
Exclusion criteriaChildren with incomplete microbiological testing or with samples without microbial detection were excluded from the analysis (Fig. 1).
DefinitionsWe defined diarrhoea as an acute episode with 3 or more watery stools lasting at least 24h. Infection by CD was defined as diarrhoea that could not be attributed to another cause and with isolation of CD or detection of CD cytotoxins from the stool sample. Cases of community-acquired ICD were defined as those in which patients had not had any contact with the healthcare system in the preceding month.
We assessed clinical characteristics by means of a scoring scale that classifies the clinical manifestations as mild, moderate or severe (Ruuska and Vesikari, Table 1).10
Scale for assessing the severity of diarrhoea in children.
| 0 | 1 | 2 | 3 | |
|---|---|---|---|---|
| Diarrhoea duration (days) | <1 | 1–4 | 5 | >6 |
| Maximum number of stools in 24h | 1–3 | 4–5 | >6 | |
| Vomiting duration (days) | 0 | 1 | 2 | 3 |
| Maximum number of vomiting episodes in 24h | 0 | 1 | 2–4 | >5 |
| Maximum body temperature (°C) | 37 | 37.1–38.4 | 38.5–38.9 | >39 |
| Degree of dehydration | No dehydration | Mild | Moderate to severe | |
| Treatment | No specific treatment | Oral rehydration | Admission, intravenous fluid therapy |
Mild: 0–8 points; moderate: 9–14 points; severe: >15 points.
Adapted from Ruuska and Vesikari.10
We also classified cases that met any of the following criteria as severe ICD11:
- 1.
Pseudomembranous colitis diagnosed by endoscopy or histopathology.
- 2.
Surgery for an ICD-related complication.
- 3.
Gastrointestinal perforation.
- 4.
Toxic megacolon.
- 5.
Pneumatosis intestinalis.
- 6.
Admission to intensive care unit (ICU) within 2 days of diagnosis.
- 7.
Shock associated to the diarrhoea.
We studied the following variables: (a) demographic characteristics: sex, age, nationality; (b) risk factors: comorbidity, gastrointestinal surgery, hospital stay within the previous month, ICU stay in the previous month, contact with healthcare system in the previous month, prior antibiotic use, type of antibiotic; (c) clinical variables: maximum body temperature, number of bowel movements per day, number of vomiting episodes per day, blood in stools, degree of dehydration; (d) laboratory values: maximum white blood cell count, maximum neutrophil count, highest C Reactive Protein (CRP) level, pH<7.3; (e) course: length of stay in days, diarrhoea and vomiting, and (f) treatment: oral or intravenous fluid therapy, antimicrobial therapy. Last of all, we analysed the microbiology test results: stool culture, stool CD culture or toxin detection test, and rotavirus and adenovirus antigen tests.
Statistical analysisWe analysed the demographic, clinical and laboratory characteristics, the risk factors and the outcomes for the different causative agents of diarrhoea. We present the results for categorical variables as frequencies or rates, and the results for continuous variables as means (SD) or medians (IQR). We compared microbial pathogens by means of the chi square test or Fisher's exact test (categorical variables); and by means of Student's t, Wilcoxon or Mann–Whitney U tests or ANOVA (continuous variables).
To determine the factors associated with CD we constructed a logistic regression model using the variables shown to be potentially correlated by the bivariate analysis. We have expressed these associations as odds ratios (ORs) with 95% confidence intervals. We performed the statistical analysis with the SPSS® Statistical software, version 20.0 (SPSS Inc; Chicago, IL; USA).
ResultsDuring the period under study, 250 hospitalised children were diagnosed with diarrhoea, 56% of which were male. Their median age was 1.2 years (IQR, 0.5–3.3).
Full microbiological testing was performed in 174 children (69.6%), and an aetiological diagnosis was made in 79 (45.4%). More than one organism was isolated in five patients: two had coinfections of CD and other microorganisms (CD+rotavirus and CD+adenovirus), and three had coinfections involving other pathogens (Campylobacter jejuni+rotavirus; Salmonella group D+C. jejuni; Salmonella group B+rotavirus+adenovirus). Children with coinfections were excluded from the analysis (Fig. 1).
Among the 74 children with a single isolate, CD was isolated in 19 (25.6%), and cytotoxin detection was positive in 13 (68.4%). Another 21 bacteria (28.6%) and 34 viruses (45.8%) were also isolated (Fig. 2).
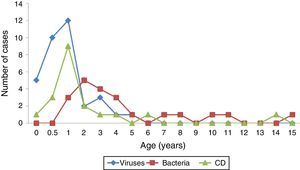
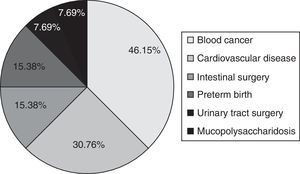
Characteristics of children infected by Clostridium difficileOf all children with ICD, 42.1% were boys (median age, 1.1 years [IQR, 0.9–2.2]). Table 2 shows the main characteristics of children with ICD and their comparison with children with diarrhoea caused by other pathogens. The age of 68.4% of children with ICD was less than 2 years (Fig. 3). The majority had a past medical history (68.4%), most frequently consisting of blood cancer, heart disease, intestinal surgery or preterm birth (Fig. 4). Community-acquired infections amounted to 15.8% of the total. Thus, 84.2% of the children had had contact with the healthcare system and 55.6% had been hospitalised in the month preceding their admission. Also, 61.1% had received antibiotics (sulfamethoxazole-trimethoprim in 4, cefotaxime in 3, amoxicillin-clavulanic acid in 1; penicillin; other in 3). Two developed severe ICD (10.52%). Enterotoxin production was confirmed in 13 patients (68.4%). We found no clinical or laboratory differences between the cases of infection by toxin producers and non-toxin producers (Table 3).
Characteristics of children with infection by Clostridium difficile compared to children with diarrhoea from other causes.
| Infection by Clostridium difficile (n=19) | Diarrhoea from other causesa (n=55) | P | Diarrhoea of bacterial aetiologyb (n=21) | P | Diarrhoea of viral aetiologyc (n=34) | P | |
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Age, median [IQR] | 1.13 [0.96–2.20] | 1.58 [0.76–3.65] | .656 | 3.49 [2.18–6.82] | .001 | 1.05 [0.54–1.54] | .126 |
| Age <1 year, % | 21.1 | 27.3 | .764 | 0 | .027 | 44.1 | .137 |
| Age <2 years, % | 68.4 | 54.5 | .291 | 18.8 | .001 | 79.4 | .507 |
| Sex, % male | 42.1 | 52.7 | .595 | 47.6 | .761 | 55.9 | .398 |
| Past medical history and epidemiology | |||||||
| Underlying disease, % | 68.4 | 3.6 | <.0001 | 0 | <.0001 | 5.9 | <.0001 |
| History of intestinal surgery, % | 5.3 | 0 | .087 | 0 | .287 | 0 | .177 |
| ICU admission in past month, % | 15.8 | 0 | .003 | 0 | .058 | 0 | .017 |
| Hospital admission in past month, % | 55.6 | 1.8 | <.0001 | 0 | <.0001 | 2.9 | <.0001 |
| Contact with health system in past month, % | 84.2 | 24.5 | <.0001 | 28.6 | .001 | 21.9 | <.0001 |
| Antibiotic use in past month, % | 61.1 | 14.8 | <.0001 | 5 | <.0001 | 20.6 | .006 |
| Clinical and laboratory characteristics | |||||||
| Temperature>38.5°C, % | 29.4 | 63.6 | .024 | 85.7 | .001 | 50 | .233 |
| Bloody diarrhoea, % | 29.4 | 22.4 | .564 | 55 | .185 | 0 | .002 |
| Diarrhoea >4 stools/day, % | 50 | 83.6 | .009 | 85.7 | .035 | 82.4 | .024 |
| Vomiting, % | 52.6 | 69.1 | .266 | 57.1 | .775 | 76.5 | .124 |
| Maximum WBC count, median [IQR] | 11.800 [6500–17,400] | 10.500 [8900–13,525] | .349 | 10.100 [9150–13,700] | .430 | 10.700 [8250–13,200] | .386 |
| Maximum neutrophil count, median [IQR] | 5.700 [3000–9500] | 6.617 [4025–9325] | .588 | 7.238 [4600–9650] | .290 | 6.300 [3200–8962] | .941 |
| Maximum CRP level, median [IQR] | 20 [1–70] | 22.5 [3–97] | .684 | 109 [45–139] | .004 | 3 [1–22.5] | .144 |
| Outcome | |||||||
| Duration of diarrhoea (days), median [IQR] | 3 [2–4] | 4 [3–5.25] | .399 | 4 [3–6.5] | .150 | 4 [2–4] | .804 |
| Length of stay (days), median [IQR] | 5 [4–19] | 4 [3–6] | .01 | 4 [3.5–5.5] | .039 | 4 [3–6] | .014 |
| Moderate-severe dehydration, % | 21.1 | 30.9 | .558 | 38.1 | .311 | 26.5 | .749 |
| Severity scale, mean (SD) | 7.8 (3.68) | 12.3 (3.75) | <.0001 | 13.05 (2.74) | <.0001 | 11.76 (4.30) | .003 |
CRP, C-reactive protein; ICD, infection by Clostridium difficile; ICU, intensive care unit; IQR, interquartile range; SD, standard deviation; WBC, white blood cell.
Characteristics of children with Clostridium difficile infection by toxin-producing status of the isolate.
| Non-toxin-producing CD (n=6) | Toxin-producing CD (n=13) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, median [IQR] | 1.13 [0.65–2.63] | 1.14 [0.95–2.82] | .661 |
| Age <1 year, % | 16.7 | 23.1 | 1 |
| Age <2 years, % | 66.7 | 69.2 | 1 |
| Sex, % male | 50 | 38.5 | 1 |
| Past medical history and epidemiology | |||
| Underlying disease, % | 23.1 | 76.9 | .320 |
| History of gastrointestinal surgery, % | 0 | 7.7 | 1 |
| ICU admission in past month, % | 16.7 | 15.4 | 1 |
| Hospital admission in past month, % | 33.3 | 66.7 | .321 |
| Health system contact in past month, % | 83.3 | 84.6 | 1 |
| Antibiotics in past month, % | 33.3 | 75 | .141 |
| Clinical and analytical characteristics | |||
| Temperature >38.5°C, % | 33.3 | 27.3 | 1 |
| Bloody diarrhoea, % | 33.3 | 27.3 | 1 |
| Diarrhoea >4 stools/day, % | 33.3 | 58.3 | .620 |
| Vomiting, % | 83.3 | 38.5 | .141 |
| Maximum WBC count, median [IQR] | 11,800 [10,150–15 600] | 13,100 [5175–18 300] | .606 |
| Maximum neutrophil count, median [IQR] | 4.732 [2950–10 700] | 5.950 [3909–10 325] | .673 |
| Maximum CRP level, median [IQR] | 20 [2.0–140.0] | 15.5 [1–72.5] | .913 |
| Outcome | |||
| Duration of diarrhoea in days, median [IQR] | 3 [1–3.5] | 4 [2.75–7] | .074 |
| Length of stay in days, median [IQR] | 4 [3.5–10.5] | 8.5 [4–24.75] | .079 |
| Moderate-severe dehydration, % | 50 | 7.7 | .071 |
| Severe ICD, % | 0 | 15.4 | 1 |
| Severity scale, mean (SD) | 9.33 (4.27) | 7.38 (3.7) | .929 |
CD, Clostridium difficile; CRP, C-reactive protein; ICD, infection by Clostridium difficile; ICU, intensive care unit; IQR, interquartile range; SD, standard deviation; WBC, white blood cell.
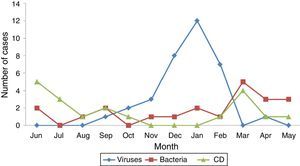
Compared to children with other isolates, a higher proportion of children with CD had chronic diseases (68.4% vs 3.6%; P<.0001) and longer lengths of stay (median, 5 vs 4 days; P=.01). From an epidemiological perspective, isolation of CD did not show a seasonal pattern, unlike other causes of diarrhoea, especially viral causes, which were most frequent in autumn and winter (Fig. 5). A higher proportion of children with ICD had had contact with the healthcare system (84.2% vs 24.5%; P<.0001), had been hospitalised (55.6% vs 1.8%; P<.0001) and had been admitted to the ICU (15.8% vs 0%; P<.003) in the preceding month. Recent use of antibiotics was also more frequent in children with ICD (61.1% vs 14.8%; P<.0001). In terms of clinical features, cases of ICD had milder symptoms, with a lower frequency of fever (29.4% vs 63.6%; P=.024), fewer bowel movements (50% vs 83.6% had more than 4 stools a day; P=.009) and lower scores in the severity scale (median, 7.8 vs 12.3; P<.0001). On the other hand, we did not find any differences in laboratory parameters.
When we compared infections by other bacteria with cases of ICD, we observed that the former occurred in older children (median, 3.4 vs 1.1 years; P=.001), with 81.2% being older than 2 years. We found no correlation between bacterial diarrhoeas and epidemiological parameters or the presence of comorbidity. These cases were the most symptomatic, with a higher frequency of fever (85.7% vs 29,4%; P=.001), fewer than 4 stools a day (85.7% vs 50%, P=.035) and higher levels of CRP (median, 109 vs 20mg/L; P=.004). The score in the clinical severity scale was higher in infections by other bacteria compared to infections by CD (median, 13.1 vs 7.8; P<.0001).
As happened with ICD, viral diseases were most frequent in younger children (median, 1.05 vs 1.1 years; P=.12), with 79.4% of cases occurring in children less than 2 years of age. Comorbidities and recent contact with the healthcare system were infrequent in these patients. As for their clinical characteristics, children with viral infections had a higher number of bowel movements than children with ICD (82.4% vs 50% had more than 4 stools/day; P=.024) and scored higher in the clinical severity scale (median, 11.76 vs 8; P=.003), although we found no differences in their laboratory values compared to those of children with ICD.
Factors associated with the isolation of Clostridium difficileIn the multivariate analysis, isolation of CD was significantly associated with the presence of comorbidities (OR 40; 95% CI, 6.8–232.3; P<.0001). Furthermore, children with ICD scored lower in the clinical assessment scale (OR 0.8; 95% CI, 0.7–0.9; P=.049).
DiscussionThis is the first study that determines the impact of ICD in children hospitalised with diarrhoea in Spain. We found that isolation of CD in these children is frequent, and that most of the isolates were toxin-producing strains. In our study, CD was typically isolated in children younger than 2 years with underlying disease, and was associated to longer lengths of stay, recent use of antibiotics and regular contact with the healthcare system. Most cases had mild courses and favourable outcomes.
In the past decades, CD has become the main cause of nosocomial intestinal infections in adults, promoted by the spread of the hypervirulent CD027 strain, which has caused outbreaks in the United States, Canada and Europe.5,12,13 The epidemiology in children reflects the changes in adults, with increasing incidences in the community and hospital settings.14–16 In Spain, there is also evidence of an increase associated with the use of antibiotics, and the older age and greater comorbidity of the current hospitalised population.17 The first case of infection by the CD027 strain in Spain was reported recently.7 In Spain, the rate of ICD in hospitalised patients is 2.4 cases per 1000 admissions,18 and no data are available for the paediatric population. In our study, the incidence of ICD in children hospitalised with diarrhoea was 3 cases per 1000 admissions, and CD was the most frequently isolated bacterium, second only to rotavirus. Community-acquired infections amounted to 15.8% of the total, compared to the 25% reported in other paediatric series.19
Still, our data do not suffice to determine whether isolation of CD in children represents true infection or asymptomatic carrier status. Asymptomatic colonisation of adults in the community occurs in 1.6–4% of the population,20 reaches 5–26% in the hospital population, and exceeds 50% during epidemic outbreaks.20,21 A paediatric study conducted in a hospital setting revealed that the situation is similar in older children, with colonisation found in 25% of the sample.19 However, colonisation in children younger than 2 years, including by enterotoxin-producing strains, exceeds 70%.2 Thus, there is evidence that colonisation by CD occurs shortly after birth and increases progressively through the first year of life,4 after which it decreases with increasing age. By age 3 years the rates of colonisation are the same as those found in adults,3 and the risk factors associated with it are underlying disease, a history of hospitalisation and exposure to more than 2 antibiotics.22 These rates of colonisation in children have important implications. First of all, children function as CD reservoirs, transmitting the disease to the adult population23,24 or to susceptible children. On the other hand, the incidental finding of CD in children with diarrhoea may be a confounder when making a diagnosis and initiating treatment. Therefore, the routine search for CD in children less than 2 years of age with diarrhoea and no additional risk factors may not be an appropriate approach, at least until we reach a better understanding of the significance of CD in this population.25
The pathogenesis of ICD in children is not well understood.3 In adults, clostridial toxins A and B bind specific colonocyte plasma membrane receptors, triggering an inflammatory response that leads to apoptosis and the clinical manifestations of colitis and diarrhoea. It is believed that, similar to what has been observed in animals,26,27 infants lack the necessary receptors for the cellular internalisation of CD toxins. Furthermore, a study detected that serum titres of antibodies to toxins A and B increased with increasing age in children less than 2 years of age, with high titres found in colonised infants.28 Last of all, breastfeeding has been demonstrated to be a protective factor against colonisation due to differences in gastrointestinal acidity,29 IgA secretion30 and competition with the breast milk flora.31,32
A relevant finding in our study was that most children with ICD had a past medical history, which was a strong risk factor for isolation of CD (OR 40, 95% CI, 6.8–232.3). The presence of comorbidities would account for the higher frequency of recent contact with the healthcare system in these children, their higher rates of hospitalisation – including stays in the ICU – and longer lengths of stay. Recent exposure to antibiotics, which is the main risk factor for ICD in adults and children,19,33 was also the most frequent factor in our study. However, since the multivariate analysis did not find an association with it, we believe that exposure to antibiotics may be a characteristic of patients with comorbidity and thus a confounding factor. Larger studies with controls are needed to analyse this relationship in depth. Another risk factor was gastrointestinal disease,19,34,35 although the association did not reach statistical significance. We did not do a proper analysis of the use of proton pump inhibitors, which has shown an association with CD in other paediatric studies.36
Our study contributes interesting information to the clinical profile of children with ICD and other causes of diarrhoea. Infection by other pathogens was typically found in older children and associated with more severe symptoms, often presenting with fever, haematochezia, increased frequency of bowel movements and laboratory abnormalities. Viral infections occurred in children younger than 2 years and presented with more severe vomiting, with no differences in the remaining variables. When we compared the course of ICD in infections by enterotoxin-producers and non-toxin producers, we found no differences in the variables under study, even though the patients with severe ICD had toxin-producing isolates. This is inconsistent with the findings of other paediatric series,37–39 although our sample was smaller. The low clinical severity found in children with ICD could be partly due to the scale used for its assessment, which was originally designed for the diagnosis of diarrhoea caused by rotavirus and is not appropriate for identifying severe cases of ICD because it does not take into account important variables associated with clinical severity in ICD. In fact, in adults, severe ICD often occurs in the absence of diarrhoea.21 Still, consistent with epidemiological studies in the paediatric age group, the low severity of ICD in children seen in our study is probably due to the presence of colonisation, rather than a true infection, among these patients.
There are limitations to our study. It has a retrospective design, which may have limited the identification of patients. However, since the incidence of CD was similar to the one described by other authors, we think it likely that most of the patients hospitalised with diarrhoea were identified. On the other hand, the retrospective design may have led to other biases, such as the inadequate identification of the variables for analysis. Secondly, we conducted the study in patients with diarrhoea, so we could not establish with certainty whether CD was acting as a pathogen or a coloniser. In addition, there are some pathogens associated with diarrhoea in children that we cannot routinely detect in our hospital, such as norovirus or enterotoxigenic E. coli. Last of all, the study was conducted in a tertiary hospital, so it may have overestimated the presence of comorbidities.
In short, our study shows that isolation of CD is frequent in children hospitalised for diarrhoea, especially among children younger than 2 years with chronic disease. The clinical picture of diarrhoea caused by CD is different from that of diarrhoea caused by other infectious pathogens, and is characterised by mild symptoms in most cases, although some of them may correspond to mere colonisations. Prospective studies of a broader scope with asymptomatic controls and conducted in the community are required to establish the true significance of CD in the paediatric population.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Santiago B, Guerra L, García-Morín M, González E, Gonzálvez A, Izquierdo G, et al. Aislamiento de Clostridium difficile en niños hospitalizados con diarrea. An Pediatr (Barc). 2015;82:417–425.
Previous presentation: This study was presented as a poster at the VI Congreso Nacional de la Sociedad Española de Infectología Pediátrica; March 2012; Bilbao, Spain.