Neonatal hypoxic-ischaemic encephalopathy due to the lack of oxygen at birth can have severe neurological consequences, such as cerebral palsy, or even the death of the asphyxiated newborn. Hypothermia is currently the only therapy included in intensive care neonatal units. This shows a clinical benefit in neonates suffering from hypoxic-ischaemic encephalopathy, mainly because of its ability to decrease the accumulation of excitatory amino acids and its anti-inflammatory, antioxidant, and anti-apoptotic effects. However, hypothermia is not effective in half of the cases, making it necessary to search for new, or to optimize current therapies, with the aim on reducing asphyxia-derived neurological consequences, either as single treatments or in combination with cooling. Within current potential therapies, melatonin, allopurinol, and erythropoietin stand out among the others, with clinical trials on the way. While, stem cells, N-acetylcysteine and noble gases have obtained promising pre-clinical results. Melatonin produces a powerful antioxidant and anti-inflammatory effect, acting as free radical scavenger and regulating pro-inflammatory mediators. Through the inhibition of xanthine oxidase, allopurinol can decrease oxidative stress. Erythropoietin has cell death and neurogenesis as its main therapeutic targets. Keeping in mind the whole scenario of current therapies, management of neonates suffering from neonatal asphyxia could rely on the combination of one or some of these treatments, together with therapeutic hypothermia.
La encefalopatía hipóxico-isquémica neonatal como consecuencia de la falta de oxígeno en el cerebro del recién nacido aparece asociada a secuelas neurológicas severas como la parálisis cerebral, pudiendo incluso desencadenar la muerte del recién nacido asfíctico. Actualmente, la hipotermia es la única terapia empleada en las unidades de cuidados intensivos neonatales, gracias, principalmente, a su capacidad para disminuir la acumulación de aminoácidos excitatorios y a su efecto antioxidante, antiinflamatorio y antiapoptótico. Sin embargo, la hipotermia no presenta beneficio clínico significativo en aproximadamente la mitad de los casos, haciéndose necesario encontrar u optimizar una terapia eficaz que disminuya las secuelas neurológicas derivadas de la asfixia perinatal, mediante su aplicación en solitario o en combinación con la hipotermia.
Dentro de estas terapias con potencial terapéutico demostrado destacan la melatonina, el alopurinol y la eritropoyetina, con ensayos clínicos en marcha, y otras como las células madre, la N-acetilcisteína o los gases nobles, con trabajos preclínicos publicados. La melatonina desarrolla un notable efecto antioxidante y antiinflamatorio, actuando como secuestrador de radicales libres y regulando diversos mediadores proinflamatorios. Por su parte, el alopurinol ejerce una potente función antioxidante mediante la inhibición de la enzima xantina oxidasa. La eritropoyetina, en cambio, tiene su principal diana terapéutica en la muerte celular y en la estimulación de la neurogénesis.
Teniendo en consideración las diferentes dianas y estrategias terapéuticas, el futuro del tratamiento de la encefalopatía hipóxico-isquémica podría hallarse en la combinación de uno o varios de estos tratamientos junto con la hipotermia terapéutica.
With an incidence of 1–3 cases per 1000 full-term births,1 hypoxic-ischaemic encephalopathy (HIE) is one of the most frequent causes of brain damage in newborns. Taking into account the growing number of high-risk births due to the increasing trends in maternal age and in the frequency of multiple pregnancies, among other factors, HIE continues to be a relevant problem in the hospital setting for which there is yet no definitive solution.
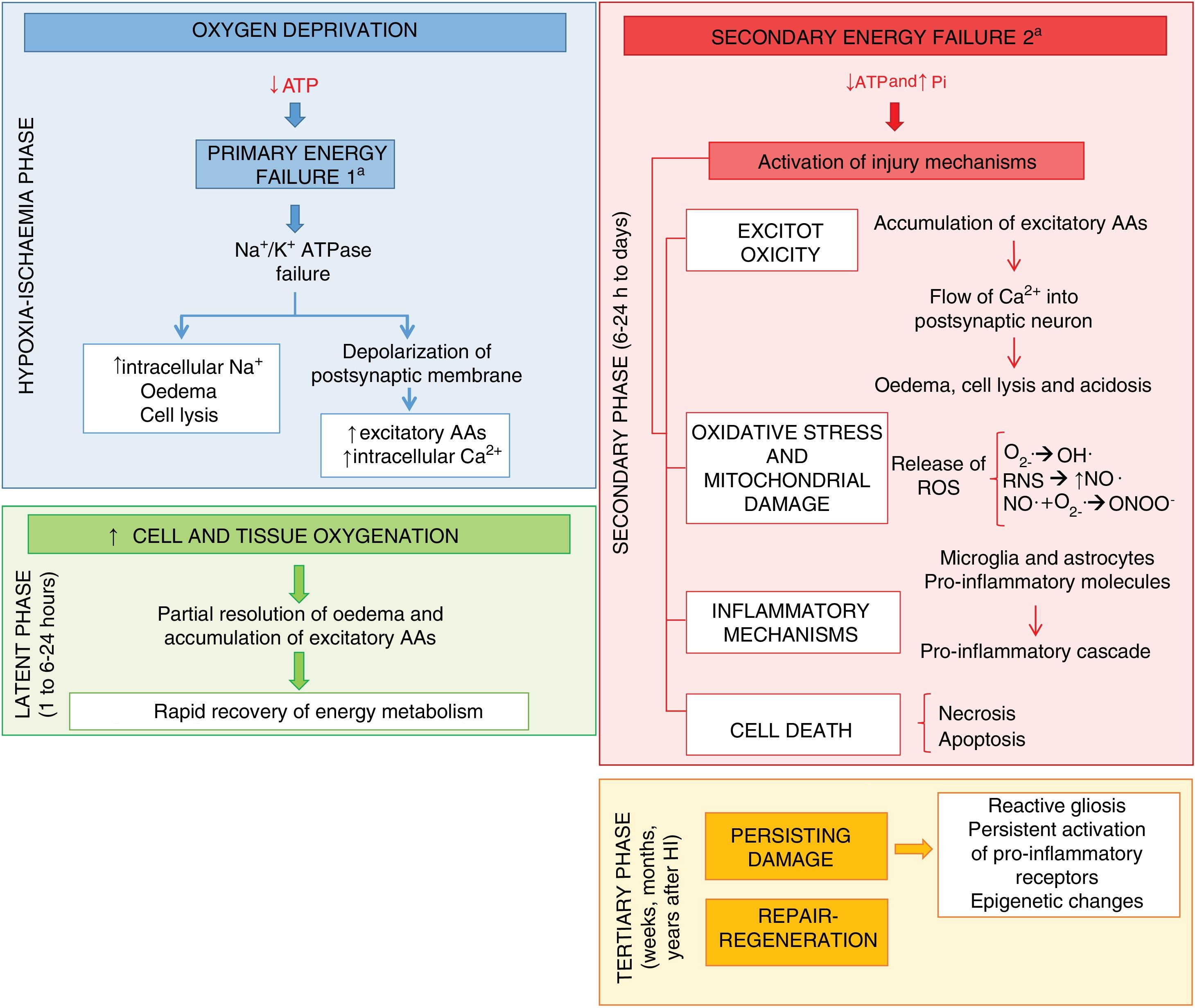
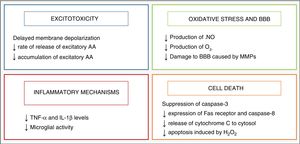
Research on the pathophysiology of perinatal asphyxia has allowed us to understand the complex process that takes place at the cellular and tissue levels as brain damage develops due to a lack of oxygen. Many of the involved mechanisms of injury are grouped based on the timing elapsed to their development, and 4 main phases have been described (Fig. 1): primary phase, or acute episode of hypoxic ischaemia (HI), latent phase, secondary phase and tertiary phase. In the primary phase, damage stems from a reduction in the delivery of oxygen to cells and tissues, which results in primary energy failure. This is followed by an apparent recovery in the levels of high-energy phosphorylated compounds, known as the latent phase. However, this improvement is only temporary and gives way to the secondary phase, when many of the pathophysiological mechanisms involved in the development of brain damage in newborns come into play, chief of which are excitotoxicity, a massive influx of calcium ions into the cells, oxidative stress, inflammation and, in most cases, cell death due to necrosis or apoptosis. Last of all, the tertiary phase is characterised by the persistence of brain damage weeks, months or even years after the initial hypoxic-ischaemic insult.
The understanding of the underlying pathophysiology of HIE has allowed the identification of potential therapeutic targets that could help reduce the brain damage caused by asphyxia and the development of numerous treatment strategies. Some of these treatments, currently in trials, are meant to work synergistically with therapeutic hypothermia, the only treatment against HIE that has been generally adopted in the neonatal intensive care setting.
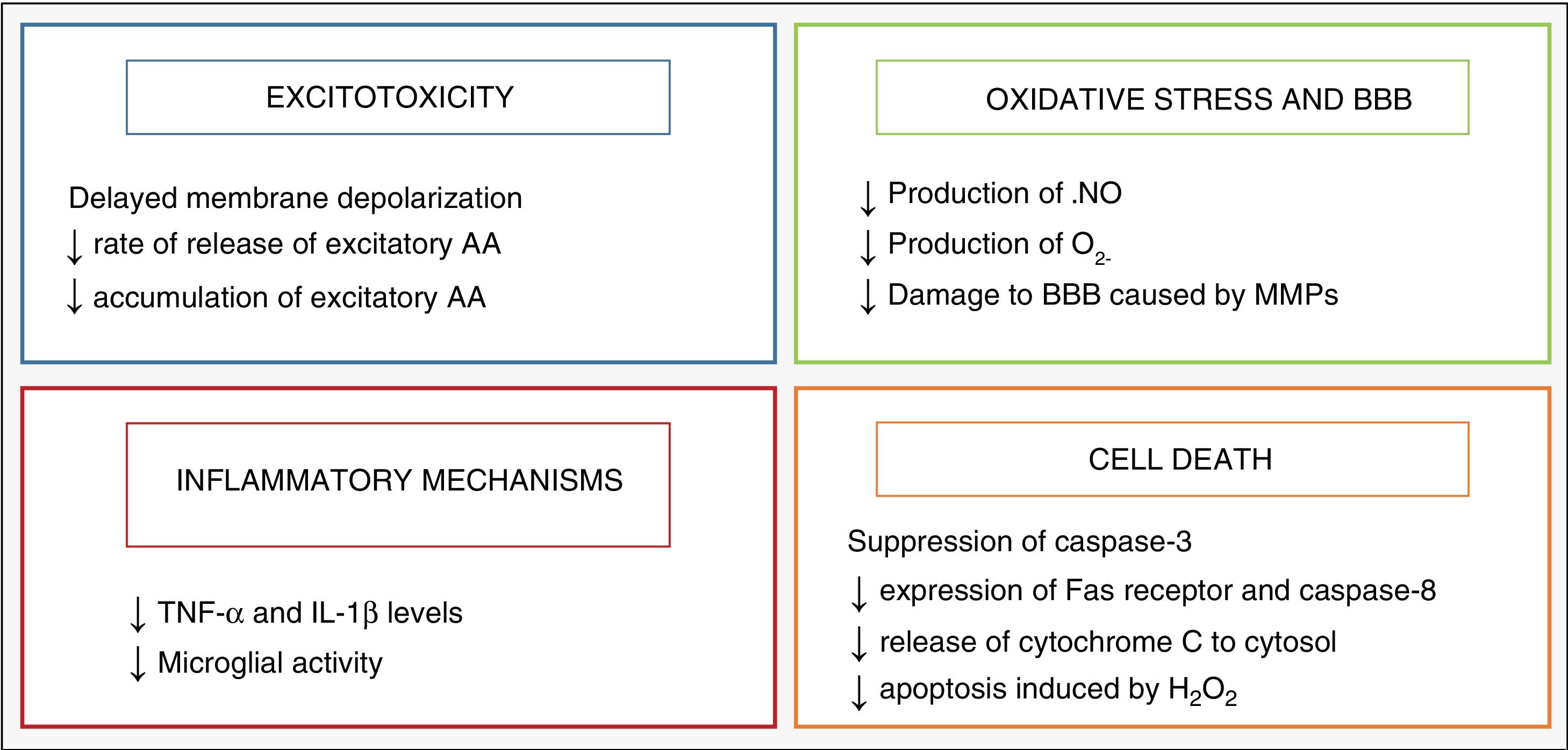
HypothermiaHypothermia started to be used as a neuroprotective strategy in infants after the discovery of an endogenous cooling mechanism that manifested in infants who had suffered some type of injury during birth.2 Its neuroprotective effects are mainly related to the decrease in the metabolic activity of the brain, of 5% with each 1°C decrease in temperature,3 which modulates some of the harmful metabolic pathways triggered by asphyxia, which are summarised in Fig. 2.
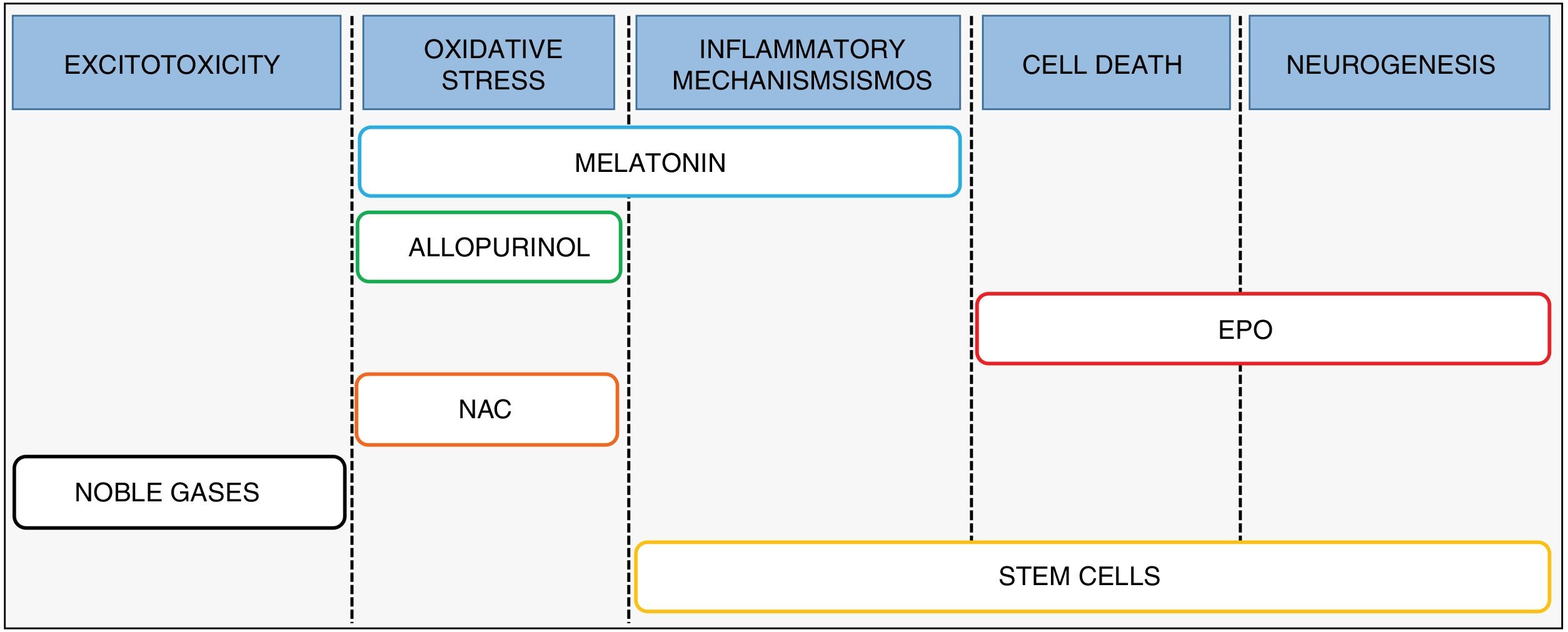
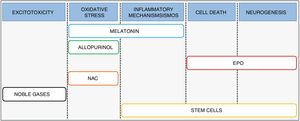
Combination therapySince the evidence suggests that current hypothermia protocols are optimal4 yet are of limited effectiveness in clinical practice,5 we need to expand are therapeutic armamentarium for the management of HIE.6 Recent research has focused on the development and implementation of treatments that may be used in combination with hypothermia, designed to act at different levels of the pathophysiologic cascade and to promote the synergy of both therapies (Fig. 3).
Melatonin (N-acetyl-5-metoxitriptamina) is a neurohormone synthesised by the pineal gland whose secretion follows a night/day cycle and whose main role is in regulating the circadian rhythm. The key aspects that allow its use in the management of HIE are its powerful antioxidant and anti-inflammatory activity7 and its ability to cross the blood–brain barrier and reach the central nervous system.8
Before being tried in newborns, melatonin has been proven to increase the level of protection afforded by hypothermia through the optimization of brain energy metabolism in a piglet model of asphyxia.9 In the clinical setting, a study published by Aly et al.10 allocated half of asphyxiated newborns to hypothermia and 5 doses of 10mg/kg/day of melatonin delivered by the oral route. The authors found a reduction in the serum levels of superoxide dismutase and nitric oxide in the patients treated with combination therapy compared to those treated with cooling alone, thus demonstrating the beneficial effects of the combination of both strategies against oxidative stress.
A recent study conducted by Balduini et al. to evaluate the safety, pharmacokinetics, dosage and effectiveness of melatonin used in combination with hypothermia found that cooling did not affect the pharmacokinetics of melatonin11 and that it was possible to obtain high serum levels of the hormone administering doses that were lower compared to those used in experimental animal models. At present, the MELPRO (NCT03806816) clinical trial is recruiting patients, aiming at a sample of 100 newborns. This and other similar studies are indispensable for the future development of phase III clinical trials and the subsequent use of melatonin in everyday clinical practice.
AllopurinolThe rationale for the use of allopurinol in the management of HIE is its inhibitory effect on xanthine oxidase, an enzyme involved in oxidative stress. In addition, this drug acts as a free-iron chelator and sequesters hydroxyl radicals.12,13 A preclinical study in rat pups where the animals were allocated to 1 of 5 groups (control group, HI group, group treated with hypothermia, group treated with allopurinol and group treated with combination therapy) found that 72h after the HI insult, the combination therapy group exhibited the lowest infarct volume.14
When it comes to its pharmacological characteristics, allopurinol can quickly cross the placenta and achieve therapeutic concentrations in newborns, as demonstrated in a study conducted on pregnant women who received 500mg of allopurinol intravenously, with evidence of optimal levels of allopurinol 5min later in umbilical cord blood samples.15 A study conducted by van Bel et al. in 1998 that analysed its potential antioxidant effect in asphyxiated newborns with severe HIE found that the intravenous administration of 40mg/kg of allopurinol achieved a reduction in the formation of free radicals.13 However, a study conducted later by Benders et al. in 2006 did not find differences between the group treated with allopurinol and the control group.16 In the conclusions, these authors identified the extreme severity of HIE in the newborns included in the sample as a possible explanation for the lack of significant differences. They also hypothesised that the period elapsed to the administration of allopurinol (3–4h after reperfusion) could have been too long to achieve favourable outcomes. In relation to the latter point, Gunes et al. administered the same dose of allopurinol given in the 2 previous studies, but within 2h from birth, and found improvements in neurodevelopmental outcomes in the treatment group.17 Along the same lines, administration of intravenous allopurinol to mothers during the delivery of foetuses with hypoxia or incipient hypoxia increased the effectiveness of treatment, reducing the cord blood levels of protein S-100β, which is a marker of brain damage.18 A clinical trial under the name Effect of Allopurinol for Hypoxic-ischemic Brain Injury on Neurocognitive Outcome (NCT03162653) is currently underway to assess the potential therapeutic effects of administration of this enzyme inhibitor in the first minutes post birth.
ErythropoietinErythropoietin (EPO) is a cytokine measuring 30.4kDa synthesised by the liver in foetal life and after birth by the kidney and the developing brain, where it acts as a growth factor and neuroprotective agent.19 The use of both EPO and recombinant human EPO (rhEPO) in HIE is based on its activity, through the engagement of EPO receptors present in neurons and glia,20 as a potent antiapoptotic agent (stimulating transcription of antiapoptotic genes BCL-2 and BCL-XL), and as an anti-inflammatory and antioxidant.19,21 In addition to its neuroprotective effect, EPO can promote long-term repair processes, such as angiogenesis, oligodendrogenesis and neurogenesis.22,23
Preclinical studies that have assessed the synergic effect of combining the administration of EPO or rhEPO with hypothermia have yielded contradictory results. In a similar rat model of hypoxic-ischaemic brain damage at day 7 post birth, Fang et al. found no significant neuroprotective effects of their combined use.24 However, in another study conducted by Fan et al.,25 the authors did observe a mild beneficial effect on sensorimotor function in the rat pups, although this difference was not reflected in the histological features of brain tissue samples.
Studies in newborns with HIE have shown that the use of rhEPO is safe at doses of 300–2500IU/kg. Low doses of rhEPO have been found efficacious in patients with moderate damage, and seem to be associated with a decreased risk of disability or death.26 Higher doses (of up to 2500IU/kg) can reduce the incidence of seizures and neurologic abnormalities at 6 months.27
Today, three phase III clinical trials are underway with a planned recruitment of a total of 840 newborns to assess the safety and efficacy of high doses of EPO (1000IU/kg) combined with hypothermia (Erythropoietin for Hypoxic Ischaemic Encephalopathy in Newborns, NCT03079167; High-dose Erythropoietin for Asphyxia and Encephalopathy, NCT02811263; Erythropoietin in the Management of Neonatal Hypoxic Ischemic Encephalopathy, NCT03163589). The main goal of the first 2 is to reduce 2-year mortality or disability, while the third will assess these two outcomes after 1 year. We await the results of these and other studies to determine whether EPO or any of its derivatives are efficacious and how they should be used in clinical practice, assessing factors such as the minimum effective dose, the route of administration, the duration of treatment, etc.
Stem cellsThe use of stem cells for treatment of all sorts of diseases, including HIE, is a field of research that continues to grow. This therapeutic approach could help repair and regenerate damaged brain tissue after the hypoxic-ischaemic insult through the interaction of stem cells with immune cells in organs distant from the brain, such as the spleen, thus altering the immune/inflammatory response. Similarly, the functional recovery achieved with their administration may be partly explained by the interaction of the transplanted cells and brain tissue, with the ensuing production of growth factors whose final effect would be reflected in increased neurogenesis and cellular proliferation.
Although we still need to deepen our knowledge to be able to use stem cells as an effective therapy, experimental studies in animals have demonstrated that different types of stem cells are able to survive in the damaged brain, differentiate into neurons or glia, integrate into the target tissue and favourably modify behavioural outcomes (reviewed in Bennet et al.28). Recent studies have reported that the administration of mesenchymal stem cells combined with 24h of cooling in rat pups 7 days post birth achieved better outcomes compared to either treatment in isolation,29 and have also found that hypothermia expands the therapeutic time window for administration of mesenchymal stem cells to up to 2 days after the hypoxic-ischaemic event.30 In addition, stem cells can regulate the immune response through their interactions with effector immune cells located in organs distant from the brain, such as the spleen, whose mobilization is known to have the potential to exacerbate the inflammatory response and ischaemic damage in the immature brain, thus enhancing their neuroprotective effect.31,32
Stem cell therapy, alone or in combination with therapeutic hypothermia, is a promising field of research that still requires clinical trials to determine, among other aspects, the most effective type of stem cells and the optimal dosage and duration of treatment to obtain the best possible treatment outcomes.28 One of the projects currently underway in the recruitment phase (Study of hCT-MSC in Newborn Infants With Moderate or Severe HIE, NCT03635450) will include 6 infants born at a gestational age of 36 or more weeks with moderate-to-severe HIE to be treated with hypothermia and 2 intravenous doses of mesenchymal stromal cells derived from umbilical cord tissue (hCT-MSC). The main objectives of this phase I trial are to assess the safety of hCT-MSC and analyse survival and neurodevelopmental outcomes in participants at 6 and 16 months, respectively. Another phase I trial (NCT00593242) obtained promising results with the autologous transplantation of umbilical cord blood cells, with 74% of the newborns that received stem cells surviving with scores of 85 or higher in the Bayley scales compared to 41% of the newborns treated with cooling alone.33
N-acetylcysteineN-acetylcysteine (NAC) is a precursor of cysteine that scavenges free radicals and involved in glutathione maintenance,34 thus regulating oxidative stress. Evidence from animal models showed a greater reduction in the cerebral infarct volume in animals treated with a combination of NAC and hypothermia compared to animals treated with only one of these interventions. Furthermore, animals treated with combination therapy showed similar outcomes in reflexes and white matter damage to those found in the control group.35 Since its administration during pregnancy does not have teratogenic effects and it can cross the placenta,36 NAC has come to be considered one of the most promising therapeutic agents for future use in neonatal intensive care units. However, to our knowledge no clinical trials have been designed to date to assess its use in the management of HIE, and the available evidence is limited to trials related to intra-amniotic inflammation, chorioamnionitis or respiratory distress syndrome.
Noble gasesNoble gases like xenon and radon have exhibited neuroprotective effects in animal models of neonatal HI. Numerous studies have analysed the possibility of using xenon as a therapeutic agent (for a review of the evidence, see the article by Lobo et al.37) due to its ability to reduce excitotoxicity after a HI insult through the modulation of NMDA glutamate receptors.38,39
The multicentre clinical trial Total Body hypothermia plus Xenon (TOBY-Xe) used xenon gas in combination with hypothermia in a sample of 92 infants born between 36 and 43 weeks of gestation. Although it did not find significant differences between groups,40 with the aim of getting more detailed information on some of the variables that may have had an impact on the outcomes of treatment with this noble gas, such as its dosage or duration, a phase II clinical trial is currently underway (CoolXenon3 Study, NCT02071394).
On the other hand, there have been no clinical trials of argon to date, but argon has been shown to improve the outcomes of cooling in terms of the levels of the N-acetyl-aspartate/lactate marker, which has been associated with increases in average cell death values and the development of neurologic sequelae in affected newborns.41 These promising results, along with its higher bioavailability and lower cost compared to xenon, make argon a molecule with a high potential for bench to bedside translation in the treatment of HIE.
ConclusionsAchieving an effective treatment for HIE is one of the great challenges facing modern medicine. For this reason, substantial efforts have been made for years to analyse the mechanisms leading to brain cell damage after perinatal asphyxia with the aim of developing effective treatments to block them. At present, paediatricians and neuroscientists are attempting to develop new compounds that could work in synergy with hypothermia with the aim of reducing to a minimum the neurologic sequelae of HIE. On the other hand, recent studies have started to include sex among the variables to take into account in the management of HIE, as dimorphic sexual differences have been found both in the mechanisms of injury (in experimental models, female animals have exhibited greater memory deficits, while male animals have showed greater susceptibility to oxidative stress), and in the pathways of the different treatments under consideration. Despite the promising advances made with the use of melatonin or compounds such as EPO, preclinical studies are still needed to further elucidate the mechanisms of action of these molecules, and clinical trials with larger samples are needed to determine the optimal dosage and routes of administration of these and other treatments.
FundingThe study was supported by a grant given by the UPV/EHU as part of the research group funding programme of the university (GIU 17/018).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cánovas-Ahedo M, Alonso-Alconada D. Terapia combinada frente a la encefalopatía hipóxico-isquémica neonatal. An Pediatr (Barc). 2019;91:59.