Infantile cerebral palsy is one of the most prevalent diseases and the most frequent cause of disability in paediatrics. Children with cerebral palsy have complex health care needs and often require the care of a multidisciplinary team. However, in many cases there is no paediatrician with overall responsibility for coordinating follow-up.
We have produced a support document intended for paediatricians coordinating the care of children with cerebral palsy. Our aim is to provide an ordered compilation of the main issues these patients may develop, to know how to identify and address them if necessary, and to establish criteria for referring these patients to other specialists.
La parálisis cerebral infantil es una de las enfermedades más prevalentes y la causa de discapacidad más frecuente en pediatría. Los niños con parálisis cerebral tienen necesidades de atención médica complejas y a menudo requieren atención por un equipo multidisciplinar, sin embargo, en muchas ocasiones no existe la figura de un pediatra responsable que coordine todo el seguimiento.
Realizamos un documento de ayuda en el abordaje de niños con parálisis cerebral dirigido a pediatras que sean coordinadores en la atención de estos pacientes. Nuestra finalidad es la de recopilar de forma ordenada los principales problemas que pueden desarrollar estos pacientes, saber cómo identificarlos y abordarlos en caso necesario, y establecer criterios para la derivación de estos pacientes a otros especialistas.
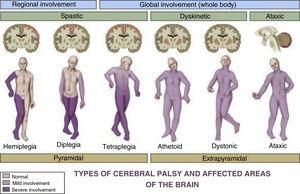
Cerebral palsy (CP) is one of the most prevalent paediatric diseases and the most frequent cause of disability in children. The term describes a group of persistent (but not unchanging) disorders of movement and posture causing activity limitation that are attributed to nonprogressive lesions, defects or interference in the developing foetal or immature brain.1 Although the definition of CP has been changing through time,2 it remains a clinical diagnosis: presence of a motor disorder causing activity limitations with evidence of nonprogressive brain damage. It is usually diagnosed after age 2 years, although most records do not include the diagnosis until age 4–5 years.1 This definition of CP encompasses a very heterogeneous group of patients (Fig. 1).3 Different scales are used to objectively assess the level of independence and functioning of children with CP4 (Table 1).
Scales used to assess the level of independence and functioning in CP.
| GMFCS | MACS | CFCS | |
|---|---|---|---|
| I | Walks without assistance, limitations in more advanced motor skills | Handles objects easily and successfully | Effective sender and receiver with unfamiliar and familiar partners |
| II | Walks with restrictions | Handles most objects but with somewhat reduced quality or speed of achievement | Effective but slower paced sender and/or receiver with unfamiliar and familiar partners |
| III | Walks with handheld assistive mobility devices | Handles objects with difficulty; needs help to prepare or modify activities | Effective sender and receiver with familiar partners |
| IV | Self-mobility with limitations, can be achieved using powered mobility | Handles a limited selection of easily managed objects in adapted situations | Sometimes effective sender and receiver with familiar partners |
| V | Patient needs to be transported by another person in a wheelchair | Does not handle objects and has severely limited ability to perform even simple actions | Seldom effective sender and receiver even with familiar partners |
The incidence of CP is of 1.5–3 cases per 1000 live births,5 and it is greater in children born preterm before 28 weeks’ gestation (111. 8/1000 live births) and with birth weights of less than 1500g (59.2/1000 live births)5.
Children with CP have complex medical needs and often require care by a multidisciplinary team, as, in addition to neurological problems, they have other disorders that increase in quantity the more severe the disease and that are key in the estimation of life expectancy.6 Families may have difficulty managing this, as they sometimes cannot find a main physician to deliver and coordinate care and have to make multiple visits to the hospital, which leads them to be dissatisfied with the care received.7
Due to the above, the figure of the care coordinator emerges as an essential element, a professional in charge of coordinating care delivery and the provision of information to the patient or health care user, acting as the main point of contact for the patient to organise care and communicate with the patient throughout the care process, without detracting from the duties of other professionals involved in care delivery.8
Material and methodsWe developed a guideline for the comprehensive care of children with CP of mild to moderate severity for primary care paediatricians in our region responsible for coordinating the care of these patients. The aim of the guideline was to propose an approach to the management of the main problems associated with CP levels I to III in the GMFCS scale and preserved motor function, inform of the most widely used drugs for each problem (Table 2) and establish criteria for the referral of these patients to other specialists (Table 3).
Drugs used most commonly in children with CP based on presenting problem and their dosage.
| Movement disorder | |
|---|---|
| Trihexyphenidyl | Initial dose: 1mg/day in 2 doses, with increases of 1mg per week until reaching the effective dose or side effects develop. High doses (>10mg/day) may be administered in 4 doses/day. Maximum of 2mg/kg/day or 70mg/day |
| Carbidopa-levodopa | Initial dose: 1mg/kg/day in 3−4 doses, with progressive weekly increases (0.5−1mg/kg) to a maximum of 10mg/kg/day. Do not use doses >4−5mg/kg/day in patients with CP |
| Spasticity | |
|---|---|
| Baclofen | 0.75−2mg/kg/day given in 3−4 doses. Gradual increase until reaching: |
| 1−2 years: 10−20mg/day in 4 doses (maximum, 40mg/day) | |
| 2−6 years: 20−30mg/day (maximum, 60mg/day) | |
| > 6 years: 30−60mg/day in 4 doses (maximum, 120mg/day) | |
| Clonazepam | Age >6 months to 10 years or to 30kg body weight: initial dose of 0.01−0.03mg/kg/day given in 2−3 doses. Slow gradual increase by 0.25−0.5mg/week to 0.1mg/kg/day up to a maximum dose of 0.2mg/kg/day |
| Age > 10 years: initial dose of 1−1.5mg/day given in 2−3 doses. May be increased by 0.25−0.5mg per week until individualised maintenance dose is reached. Maximum dose of 20mg/day | |
| Tizanidine | 0.1−0.2mg/kg/day given in 2−3 doses. Generally, the recommended initial doses are: |
| 18 meses-7 years: 1mg/day at night | |
| 7−12 years: 2mg/day in 1−2 doses | |
| >12 years: dosage similar to adults, starting with 4mg/day given in 2 doses (to a maximum of 36mg/day) | |
| Gastro-oesophageal reflux | |
|---|---|
| Omeprazole | 0.6−3.5mg/kg/day |
| Baclofen | 0.7mg/kg/day. Consider in case of associated spasticity |
| Constipation | |
|---|---|
| Polyethylene glycol | Initial disimpaction: 1,5mg/kg/day in 1 or 2 doses |
| Maintenance: 0.8mg/kg/day in 1 or 2 doses | |
| Lactulose | 1−2ml/kg/day in 1 or 2 doses |
| Bone health | |
|---|---|
| Calcium | 1−3 years: 500mg/day |
| 4−8 years: 800mg/day | |
| > 8 years- 18 years: 1300mg/day | |
| Vitamin D | Age < 1 year: 800 IU-1000 IU |
| Age≥year: 800 IU-4000 IU | |
| Bladder dysfunction | |
|---|---|
| Oxybutynin | 0.1−0.4mg/kg/day (maximum 15mg/day) |
| Desmopressin | 120−240μg/day 30min before bed |
| Drooling | |
|---|---|
| Glycopyrronium bromide | 1 month-17 years: initial dose of 0.02mg/kg every 12h. In case of poor response, it can be given every 6−8h. The dose can later be increased by 0.02mg/kg/dose to 0.1mg/kg/dose. Maximum dose, 0.1mg/kg/dose or 2mg/dose |
| Scopolamine | Apply patches under or behind the ear. First week: ¼ patch, second week: ½ patch, third week: ¾ patch, fourth week: full patch. Change every 3 days, alternating ears: |
| Neonates > 32 semanas-2 years: ¼ patch every 72 h | |
| 3−9 years: ½ patch every 72h | |
| >10 years: 1 patch every 72h | |
| Trihexyphenidyl | Initial dose: 0.1mg/kg/day in 3 doses, in case of a weak effect, increase progressively in weekly steps to 0.5mg/kg/day (maximum dose, 10mg/day) |
| Sleep disturbance | |
|---|---|
| Melatonin | 3−15mg/day |
| Lorazepam | 0.05−0.1mg/kg/dose (maximum 2−4mg/dose) |
| Zolpidem | Age > 2 years: 0.25mg/kg/day (maximum 5−10mg) |
Proposed management, diagnostic tests and referrals to specialised care.
| Followup by coordinating paediatrician | Diagnostic tests | Referral to specialised care | ||
|---|---|---|---|---|
| Always | Based on condition | |||
| Neurologic disorders | Assess for epileptic seizures, intellectual disability, neuropsychiatric problems, movement disorders, language/speech disorders and spasticity | EEG in case of suspected epileptic seizures | Neurology | |
| Assist with integration and learning in school | ||||
| Orthopaedic disorders | Assess for fixed contractures and osteoarticular deformities | Rehabilitation | Traumatology/Neurosurgery in case of: | |
| Early intervention | Orthopaedic complications refractory to first-line treatment | |||
| Surgical treatment of spasticity | ||||
| Gastrointestinal disorders | Identify the caregiver in charge of feeding patient and ask how feeding is performed. Direct observation of mealtimes. Food frequency questionnaire. Specifically ask about symptoms related to: | Complete blood count, serum iron, ferritin, transferrin, calcium, magnesium, phosphate, albumin, total protein, liver enzymes, vitamins A, B12, D, E, folic acid, parathyroid hormone and zinc every year | Gastroenterology in case of: | |
| GOR | Undernutrition | |||
| Dysphagia | Suspected dysphagia | |||
| Constipation | GOR or constipation refractory to treatment | |||
| Anthropometric evaluation: | ||||
| < 2 years: every 1−3 months | ||||
| > 2 years: every 3−6 months | ||||
| Combined use of specific growth charts for children with CP for sex and GMFCS level and WHO growth standards | ||||
| Bone health disorders | Food frequency questionnaire (calcium and vitamin D) every 6−12 m | Spine radiograph at 6−8 years, and every 2 years thereafter | Rheumatology in case of: | |
| DEXA at 6 years if GMFCS level IV-V or younger with risk factors | Osteoporosis | |||
| In case of supplementation with calcium and vitamin D, measure calcium, ionic calcium, phosphate, parathyroid hormone, vitamin D and alkaline phosphatase every 6 months | Low BMD for age | |||
| Dental health | Regular checkups including oral examination and education on oral hygiene | Odontology in case of: | ||
| Caries | ||||
| Gumboils | ||||
| Gingivitis | ||||
| Malocclusion | ||||
| Respiratory disorders | Look for warning signs | Annual acid-base status | Pulmonology in case of: | |
| 6 years: lung function test | ||||
| Recurrent pneumonia | ||||
| Persistent wheezing | ||||
| Suspected sleep apnoea-hypopnoea syndrome | ||||
| Rehabilitation: | ||||
| Recurrent atelectasis | ||||
| Ear-nose-throat (ENT): | ||||
| Obstructive apnoea | ||||
| Impaired vision | Look for warning signs | Ophthalmology | ||
| Assessment with PREVIAS questionnaire (age < 2 years) and Dutton’s Visual Skills Inventory (age > 5 years) | ||||
| Hearing loss | Complaint-directed history (family history of hearing loss, aetiology of CP, use of ototoxic drugs). Education of parents and teachers on hearing behaviour and language development. | ENT in case of: | ||
| Look for warning signs | Warning signs | |||
| Recurrent acute otitis media (AOM) or persistent serous AOM | ||||
| Urinary disorders | Complaint-directed history (bowel habits, urinary habits, history of urinary tract infection) | Yearly workup. Renal function: urea and creatinine | Urology in case of: | |
| Look for warning signs: | Recurrent urinary tract infection | |||
| Symptom diary: fluid intake, number and volume of voidings, bowel movements and incontinence episodes | Warning signs | |||
| Management of sexual health | ||||
| Drooling | Assess with Thomas-Stonell and Greenberg and Drooling Impact scales | |||
| Sleep disturbances | Sleep diary | |||
| Pain | Routine assessment of pain and exploration of pain triggers | |||
| Assess with r-FLACC scale | ||||
| Psychosocial support | Inform primary care nurse | Social worker | ||
| Child and adolescent mental health services | ||||
| Care by paediatric palliative care and chronic complex disease team | Refer in case of: | |||
| Clinical inflection point | ||||
| Symptoms that cannot be controlled with usual treatment | ||||
| Highly vulnerable patients with complex needs | ||||
| Difficulty with decision-making, need of guidance in treatment planning | ||||
| At discretion of paediatrician | ||||
Epilepsy, found in 35%–62% of children with CP,9 is more frequent in children with abnormal neuroimaging findings and greater motor impairment. Nonepileptic paroxysmal events and some movement disorders may create confusion and errors in treatment. Performance of an electroencephalogram (EEG) is indicated in case epileptic seizures are suspected. In this group of patients, the most frequent disorder is status epilepticus (14%–47% of children with CP and epilepsy), and therefore seizures must be treated at an early stage. The management of epilepsy does not differ from the followup in patients without CP, but in follow-up visits, clinicians should always ask about seizures or any “new”, “different” “recurrent” events, uncontrolled movements etc, to identify epilepsy that has gone unnoticed by the parents. Videos may be very useful to understand the presentation of these events and determine whether performance of EEG is indicated.
Intellectual disability (ID), observed in 40%–70% of individuals with CP,10 is strongly associated with the type of CP (more severe ID in spastic versus dyskinetic CP and in quadriplegia versus hemiplegia), the presence of epilepsy and abnormal features on EEG or neuroimaging.
The most frequent language disorders are dysarthria (in 40%) and inability to talk (in 25%).11 Patients with CP may also experience impairment in other areas of communication, such as the development of gestures and facial expressions, receptive and expressive language acquisition and speech production. Personalised treatment must be initiated early (ideally starting before age 2 years) in any child at risk of CP.
Over 50% of patients with CP have mental health disorders,12 chiefly mood and conduct disorders, social impairment, attention deficit and hyperactivity which, added to the other features of the disease, exacerbate problems with schooling and adaptation. On the whole, they cause significant anxiety and hinder integration of the child in the community, so it is important to identify these disorders, not underestimate them and offer any resources that may be needed. Followup by school counsellors, adaptations at the level required by the child and support from a speech therapist or speech-language pathologist (SLP) or educational therapist (ET) are indispensable in these patients. These children are more likely to be the target of bullying or harassment, so it is important to watch for red flags that may indicate their presence.
Movement disorders (dystonia, chorea, athetosis and ballism), described in up to 40% of patients,13 are more frequent in dyskinetic CP and often associated with spasticity. They cause significant functional impairment, as they may interfere with, impede or possibly preclude performance of activities of daily living (ADLs), and may also cause pain. The most frequent one is dystonia, characterised by involuntary muscle contractions, sustained or intermittent, that cause twisting movements or abnormal posture caused by the simultaneous contraction of agonist and antagonist muscles. Physical therapy is the cornerstone of treatment. Trihexyphenidyl is usually the first-line drug used in pharmacotherapy.
Spasticity occurs in 85% of children with CP14 and results in functional impairment in ADLs (walking, eating, washing, dressing), in addition to possibly causing muscle pain, spasms and abnormal postures. In some cases, spasticity may be beneficial, as the increased muscle tone in the lower limbs helps maintain the standing position, contributing to the maintenance of muscle mass and bone density. Spasticity can be measured with the modified Ashworth scale and the Tardieu scale (Table 4). A personalised treatment plan must be developed, with realistic goals established in agreement with the patient and caregiver. Possible goals may include optimising functioning, improving hygiene, alleviating pain or preventing complications to improve the quality of life of the patient and the family. The coordinating paediatrician must know the different treatment options: nonpharmacological treatments such as physical therapy, occupational therapy and assistive devices, oral pharmacological treatment including oral therapy with baclofen (first-line treatment for generalised spasticity) or local parenteral treatment with botulinum toxin, and surgical intervention.
Modified Ashworth scale and Tardieu scale.
| Modified Ashworth scale | Tardieu scale | |
|---|---|---|
| 0 | No increase in tone | No resistance throughout passive movement |
| 1 | Slight increase in tone giving a catch when slight increase in muscle tone, manifested by the limb was moved in flexion or extension | Slight resistance throughout, with no clear catch at a precise angle |
| 1+ | Slight increase in muscle tone, manifested by a catch followed by minimal resistance throughout the range of motion | |
| 2 | More marked increase in tone but more marked increased in muscle tone through most limb easily flexed | Clear catch at a precise angle followed by release |
| 3 | Considerable increase in tone, passive movement difficult | Fatigable clonus (< 10s) occurring at a precise angle |
| 4 | limb rigid in flexion or extension (abduction, adduction, etc) | Unfatigable clonus (> 10s) occurring at a precise angle |
Most are secondary to spasticity, which creates fixed contractures that give rise to osteoarticular deformities (thumb-in-palm, wrist and elbow flexion, scoliosis, hip displacement/dislocation, clubfoot) that worsen the clinical condition of the child and may require orthopaedic surgery.15
Gastrointestinal disordersBetween 80% and 90% of patients with CP have chronic gastrointestinal disorders,16 chiefly, on one hand, malnutrition and impaired growth, and on the other, the main associated gastrointestinal disorders: dysphagia, gastro-oesophageal reflux (GOR) and constipation.17
The aetiology of malnutrition in children with CP is multifactorial, and this problem is identified in 60%–90% of affected patients. The factors underlying undernutrition are decreased intake, micronutrient deficiency and in rare cases increased nutritional requirements. The energy requirement can be estimated using the Schofield equation, as done in the general population, although it is important to take into account that non-ambulatory children that depend on assistive devices may have an energy expenditure that is 70% the expenditure observed in their healthy peers.
Dysphagia affects between 40% and 90% of children with CP.18 The development of oral motor skills depends on neural maturation and requires the coordination of muscle groups that are under the control of cranial nerves, the brainstem and the cerebral cortex. Children with CP have weak sucking, poor suck-swallow coordination, inefficient lip closure and ineffective chewing that often results in an increased duration of feeding and in some cases an insufficient energy intake, which would result in malnutrition. On the other hand, oropharyngeal dysphagia carries a risk of bronchopulmonary aspiration of food that can worsen the respiratory condition of the patient. Therefore, assessing the safety and effectiveness of oral feeding is of vital importance. The assessment should include questions about the duration of meals (with durations > 30min considered a possible warning sign) or the development of cough, cyanosis, sweating or dyspnoea while eating.
The aetiology of GOR is multifactorial: abnormal posture, dysmotility due to neurologic impairment with increased frequency of transient lower oesophageal sphincter relaxation and increased abdominal wall pressure secondary to spasticity and scoliosis. Gastro-oesophageal reflux is found in nearly half of children with CP and should be suspected in patients with food refusal, vomiting, drooling, respiratory complications or other nonspecific symptoms such as irritability, anaemia or dental abnormalities. In mild cases, clinicians ought to recommend thickening any consumed fluids and staying upright after meals. If this does not achieve control of symptoms, proton pump inhibitors are the first-line agents used for pharmacological treatment.19
Lastly, posture and muscle tone abnormalities, deficient fluid intake, immobility or the use of pharmaceuticals lead to constipation in approximately half of patients with CP. The initial approach to its management is disimpaction (at which time, foods with a high fibre content should be avoided), followed by maintenance therapy with polyethylene glycol or lactulose in addition to dietary measures (adequate intake of fibre and fluids).16
Bone diseaseChildren with CP are at risk of low bone mineral density (BMD) and osteoporosis. Immobility, inadequate intake of micronutrients (calcium and vitamin D), undernutrition and anticonvulsant therapy may contribute to their development. Pathologic fractures may occur in up to 20% of these patients, most frequently involving the distal femur. Early detection is important, as most cases are asymptomatic (up to 80% of vertebral fractures).
Dual-energy x-ray absorptiometry (DEXA) is the most widely used technique to measure BMD. Low BMD is defined as a BMD z-score for age of less than –2 (adjusted for height if the height z-score of the patient <–2). The diagnosis of osteoporosis requires presence of low BMD combined with a history of clinically significant fractures (2 or more long bone fractures by age 10 years or 3 or more long bone fracture at any age up to 19 years) or vertebral compression fractures in the absence of local disease or high-energy trauma.
When it comes to the measures used to improve BMD, the current evidence shows that calcium and vitamin D supplementation may help and that bisphosphonates probably help, while there is insufficient evidence to support high-impact physical activity (running, jumping) for this purpose.
Oral calcium supplementation in children with CP with a normal BMD and without bone fragility should be considered if calcium intake is insufficient, although it is always preferable to improve calcium intake through dietary sources. In patients with low BMD, bone fragility or osteoporosis, supplementation with calcium and vitamin D is indicated.19
Oral health problemsChildren with CP are more likely to develop caries, malocclusion and periodontal disease compared to the general population (incidence of up to 90%).20
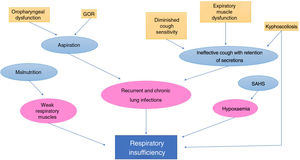
Respiratory disordersThey are one of the main causes of morbidity and mortality in children with CP. Many factors are at play in respiratory complications in patients with CP21 (Fig. 2).22
Respiratory manifestations vary with the age of the patient, and it is more frequent for infants to experience difficulty feeding, aspiration and apparent life-threatening events and for older children to experience persistent cough, noisy breathing and recurrent respiratory infections. It is also important to be watchful for manifestations suggestive of sleep apnoea-hypopnoea.
Impaired visionBetween 40% and 75% of children with CP have some degree of visual impairment.23 Clinicians must be alert to recognise the signs of possible vision impairment: nystagmus (fusion maldevelopment nystagmus or lasting 15–30s in vesticulo-ocular reflex response tests), unusual behaviour, absence of ocular reflexes, purposeless eye movements and lack of visual curiosity and attention.24,25
Hearing lossThe reported prevalence of hearing loss in children with CP ranges from 4% to 13%.25 In children with CP, the newborn hearing screening should include otoacoustic emissions and an auditory brainstem response test. Red flags of hearing loss include poor response to auditory stimuli, unusual behavioural responses and abnormal language development.
Genitourinary problemsUp to 60% of patients with CP experience voiding dysfunction, enuresis, urinary urgency, incontinence or neurogenic bladder.26 The most specific warning signs are constant dripping of urine, need of abdominal contraction for initiation of urination or weak urine stream and polydipsia.
In all patients, treatment focuses on pursuing continence, regardless of disease severity, through behavioural and dietary measures (fluid intake of 1500mL/m2, treatment of constipation, control of schedules and posture, enuresis alarm, etc), pharmaceuticals such as oxybutynin in case of incontinence secondary to detrusor hypertonicity or desmopressin once daytime continence is achieved and nocturnal enuresis persists, intermittent catheterization or injection of botulinum toxin.27
DroolingThe reported prevalence of drooling ranges from 10% to 58%.28 In the assessment of affected patients, clinicians should determine its frequency, severity and impact on the quality of life of children and their caregivers.
The main goals of treatment of drooling are: reducing its impact on physical, social and mental health, improve quality of life for patients and caregivers and reduce the burden experienced by caregivers.
Treatment consists of:
- -
Teaching sensory awareness and oral motor skills: this is the cornerstone of management for children able to follow directions and cooperate with treatment.
- -
Pharmacotherapy: to reduce production of saliva. The first-line agents are trihexyphenidyl (the drug of choice for dyskinetic forms of CP), glycopyrronium bromide or scopolamine, with atropine reserved for second-line treatment.
- -
Botulin toxin infiltration.
- -
Surgery.
Sleep disorders are found in 25% of children with CP compared to 5% of the general population. The main sleep disorders in these patients are difficulty falling and staying asleep, difficult morning awakening, nightmares and sleep anxiety.29 In many cases, these problems are due to environmental factors, hunger or thirst, and in many others to respiratory problems, need for repositioning due to immobility, seizures, pain or drug side effects, among other reasons.
The mainstay of treatment is sleep hygiene. If a treatable cause cannot be identified, melatonin can be tried out, especially in patients that have difficulty falling asleep, avoiding routine use of sedatives.
PainPain is a frequent symptom, and some studies report a prevalence of up to 32% in children30 and 74%31 in youth with CP. The identification, assessment and management of pain in children with CP continues to pose a significant challenge mainly due to deficient exploration during the history taking, communication problems in the patients and the limited availability of instruments to measure pain. Thus, clinicians must routinely assess the presence of pain and its possible triggers.
While there is no consensus on which instrument is best to assess pain, of all that are currently available we recommend the revised Face, Legs, Activity, Cry, Consolability (r-FLACC) scale32 due to its ease of use and feasibility.
The most frequent mechanisms33 that generate pain in children with CP include nociceptive—somatic (spasticity, hip subluxation, fracture, etc) or visceral (constipation, GOR, gastric ulcer, etc)—and neuropathic, and we must not forget procedural pain (physical therapy, botulin toxin injections, etc). Children with CP often experience pain from several sources.
When it comes to pain management, the first step should be a comprehensive evaluation to attempt to identify the cause and treat it. If a cause cannot be established, clinicians should consider a stepped-approach trial of simple analgesia (such as paracetamol and/or ibuprofen) for mild to moderate pain.34
Psychosocial supportEstablish a care plan for the patient that includes support for caregivers and education to teach them the necessary skills to play an active role in the lifelong development of the individual with CP. To this end,
- -
Inform the primary care nurse of the case.
- -
Refer to social worker at the primary care centre to make initial contact with community-based social services.
- -
Determine the need to refer to community-based child and adolescent mental health services on a case-by-case basis.
Having discussed the different comorbidities found in children with CP, we must not neglect to highlight the fragile condition of these patients, so that while they may be stable for periods of variable duration, they are more vulnerable to experiencing decompensations due to intercurrent conditions that exacerbate the underlying disease. Therefore, it is important to take these resources into and make the necessary referrals in order to adjust treatment goals to the best interest of the patient in each situation.
ConclusionsThis document highlights the complexity of care in this group of patients and aims to facilitate care delivery by primary care paediatricians.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cantero MJP, Medinilla EEM, Martínez AC, Gutiérrez SG. Abordaje integral del niño con parálisis cerebral. An Pediatr (Barc). 2021;95:276.