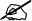The number of people with immunosuppression is increasing considerably due to their greater survival and the use of new immunosuppressive treatments for various chronic diseases. This is a heterogeneous group of patients in whom vaccination as a preventive measure is one of the basic pillars of their wellbeing, given their increased risk of contracting infections. This consensus, developed jointly by the Sociedad Española de Infectología Pediátrica (Spanish Society of Pediatric Infectious Diseases) and the Advisory Committee on Vaccines of the Asociación Española de Pediatría (Spanish Association of Paediatrics), provides guidelines for the development of a personalised vaccination schedule for patients in special situations, including general recommendations and specific recommendations for vaccination of bone marrow and solid organ transplant recipients, children with inborn errors of immunity, oncologic patients, patients with chronic or systemic diseases and immunosuppressed travellers.
El número de personas con inmunodepresión está aumentando considerablemente debido a su mayor supervivencia y al empleo de nuevas terapias inmunosupresoras en diversas patologías crónicas. Se trata de un grupo heterogéneo de pacientes en los que la vacunación como arma preventiva supone uno de los pilares básicos de su bienestar, por su elevado riesgo a padecer infecciones. Este consenso, elaborado conjuntamente entre la Sociedad Española de Infectología Pediátrica (SEIP) y el Comité Asesor de Vacunas de la Asociación Española de Pediatría (CAV-AEP), aporta unas directrices para programar un calendario adaptado a cada paciente en situaciones especiales que incluye recomendaciones generales, vacunación en pacientes con trasplante de médula y trasplante de órgano sólido, vacunación en niños con errores innatos de la inmunidad, vacunación en el paciente oncológico, vacunación en pacientes con enfermedades crónicas o sistémicas y vacunación en niños viajeros inmunodeprimidos.
In recent decades, the number of people living with immunosuppression has been increasing due to their increased survival and the use of new immunosuppressive treatments for various chronic diseases. This is a heterogeneous group of patients in whom immunosuppression may result from an inborn error of immunity or secondary to a disease or immunosuppressive therapy.
Depending on the cause of immunosuppression (Table 1), the course of underlying disease and the treatments use, the level of immune impairment can be mild, moderate or severe, and it can also remain stable, vary or reverse over time.1–3
Classification of immunodeficiencies based on the extent of immunosuppression.
| High-level immunosuppressiona |
|---|
| • Inborn errors of immunity involving B and T cells |
| • Chemotherapy and/or radiotherapy in the past 6 months |
| • HSCT recipients |
| • CAR-T cell therapy recipients |
| • Solid organ transplant recipients |
| • Chronic renal insufficiency. Renal replacement therapy (haemodialysis and peritoneal dialysis) |
| • HIV infection with CD4 count <200 cells/mm3 in patients aged ≥14 years or CD4 percentage <15% in patients aged <13 years |
| • Treatment con: azathioprine >3 mg/kg/day, 6-mercaptopurine >1.5 mg/kg/day, methotrexate >0.4 mg/kg/week or ≥15 mg/m2/week or ≥25 mg/week, leflunomide ≥0.5 mg/kg/day or >20 mg/week, ciclosporin >2.5 mg/kg/day, mycophenolate mofetil ≥30 mg/kg/day or >1000 mg/day, cyclophosphamide oral >2 mg/kg/day, tacrolimus >1.5 mg/day. |
| • Steroid therapyb: systemic treatment with prednisone (or dose equivalent): ≥2 mg/kg/day for ≥14 days; ≥1 mg/kg/day for ≥28 days; ≥20 mg/day in patients weighing more than 10 kg for ≥14 days. Intravenous methylprednisolone, bolus ≥500 mg. |
| • Immunosuppressive monoclonal antibodies (MABs)c and other biologic agents: anti-TNF-α infliximab, adalimumab, certolizumab, etanercept and golimumab ), B cell depletion therapy (rituximab), T cell depletion therapy (alemtuzumab), interleukin inhibitors including IL-6R (sarilumab, tocilizumab), IL-17A (secukinumab), IL-12/23 (ustekinumab), IL-23 (guselkumab), IL-1 (canakinumab), IL-1R (anakinra), IL-4/13 (dupilumab), IL-5 (mepolizumab) and anti-B-cell activating factor (BLyS, belimumab). Janus kinase (JAK) inhibitors. |
| • Combination therapy, independently of the dose. |
| Low-level immunosuppression |
|---|
| • Asymptomatic HIV infection with CD4 count ≥200 cells/mm3 in patients aged ≥14 years, or a CD4 percentage ≥15% in patients aged <13 years. |
| • Steroid therapy ≥14 days with a single dose/day below the dose that produces high-level immunosuppression or patients receiving steroids on alternating days. |
| • Methotrexate at a dose ≤0.4 mg/kg/week or <15 mg/m2/week, azathioprine at a dose ≤3 mg/kg/day or 6-mercaptopurine at a dose ≤1.5 mg/kg/day. |
Adapted from the American Academy of Pediatrics Red Book 2023.
Steroid therapy: treatments lasting <2 weeks (except boluses), lower doses, replacement therapy at physiological doses, topical treatment, or inhaled or intra-articular steroid administration are not a contraindication for administration of live attenuated vaccines. The interval required after high-level immunosuppressive therapy for the administration of attenuated vaccines is 1 month, and 3 months after a bolus.
Immunosuppressive MABs: administer vaccines before treatment initiation, at least 2 weeks before for inactivated vaccines and 4 weeks before for attenuated vaccines 4 weeks (6 weeks before treatment with alemtuzumab). During treatment, if administration of an inactivated vaccine is necessary, it is best to administer it a few days before the next MAB dose, and attenuated vaccines are contraindicated, although their administration can be contemplated on a case-by-case basis in stable patients receiving maintenance immunosuppressive therapy (section 5). After treatment completion, the recommended interval before vaccine administration is of 5 times the plasma half-time of the drug, which amounts to 3–6 months after treatment with anti-TNFα or anti-interleukin antibodies, and 12 months following B cell depletion.
Immunosuppressed individuals are at increased risk of contracting vaccine-preventable diseases that can cause exacerbations of underlying disease and have a more severe course compared to immunocompetent individuals. Therefore, it is of utmost importance to optimise vaccination in this population.4 In the context of immunosuppression, the immune response to vaccines is suboptimal, so vaccination should be performed at the earliest opportunity to achieve the best possible response, preferably at the time of diagnosis, but ensuring that necessary treatment is not deferred. However, misinformation and fears concerning the safety of vaccines or the course of underlying disease frequently result in undervaccination or delayed vaccination in immunosuppressed children.5
This document, developed through the critical review of relevant scientific information obtained through a non-systematic review of published research and expert consensus documents, provides guidelines to develop a personalised vaccination schedule for each patient. It is divided in 6 sections: (a) general recommendations, (b) vaccination of haematopoietic stem cell transplant (HSCT) and solid organ transplant (SOT) recipients, (c) vaccination of children with innate errors of immunity, (d) vaccination of oncological patients, (e) vaccination of patients with chronic or systemic diseases and (f) vaccination of immunosuppressed children who plan to travel.
General recommendations for vaccination of immunosuppressed children and adolescentsWith the exceptions allowed for special situations, immunosuppressed children and adolescents should receive all the vaccines in the routine immunization schedule in addition to those recommended to the specific group to which they belong. The annual vaccine against the seasonal flu, the pneumococcal vaccine and the SARS-CoV-2 vaccine are all recommended for immunosuppressed children in general, to be given according to the current schedule for risk groups.
Inactivated vaccines do not pose a threat to the health of immunosuppressed patients, although the immunogenicity will not be optimal and they may require additional primary series and/or booster doses.
Live attenuated vaccines (measles, mumps and rubella [MMR], varicella, rotavirus, attenuated influenza, oral typhoid fever, yellow fever, bacillus Calmette–Guérin [BCG] and oral polio) are contraindicated in severely immunosuppressed patients (see Table 1) due to the risk of replication and development of disease by vaccine strains. Recent data have shown that these vaccines can be safe and immunogenic if the markers of humoral and cellular immunity are within specific ranges, but further studies are required to confirm the recommendation of these vaccines under these circumstances.6
In the case of elective immunosuppression, administration of all indicated vaccines with an accelerated schedule is recommended before initiation of immunosuppression, administering live attenuated vaccines at least 4 weeks before and inactivated vaccines at least two weeks before. After completion of treatment, the recommended interval prior to vaccination ranges from 3 months to 2 years depending on the specific situation and type of vaccine. Table 2 summarises the general recommendations for vaccination of immunosuppressed children and adolescents.7
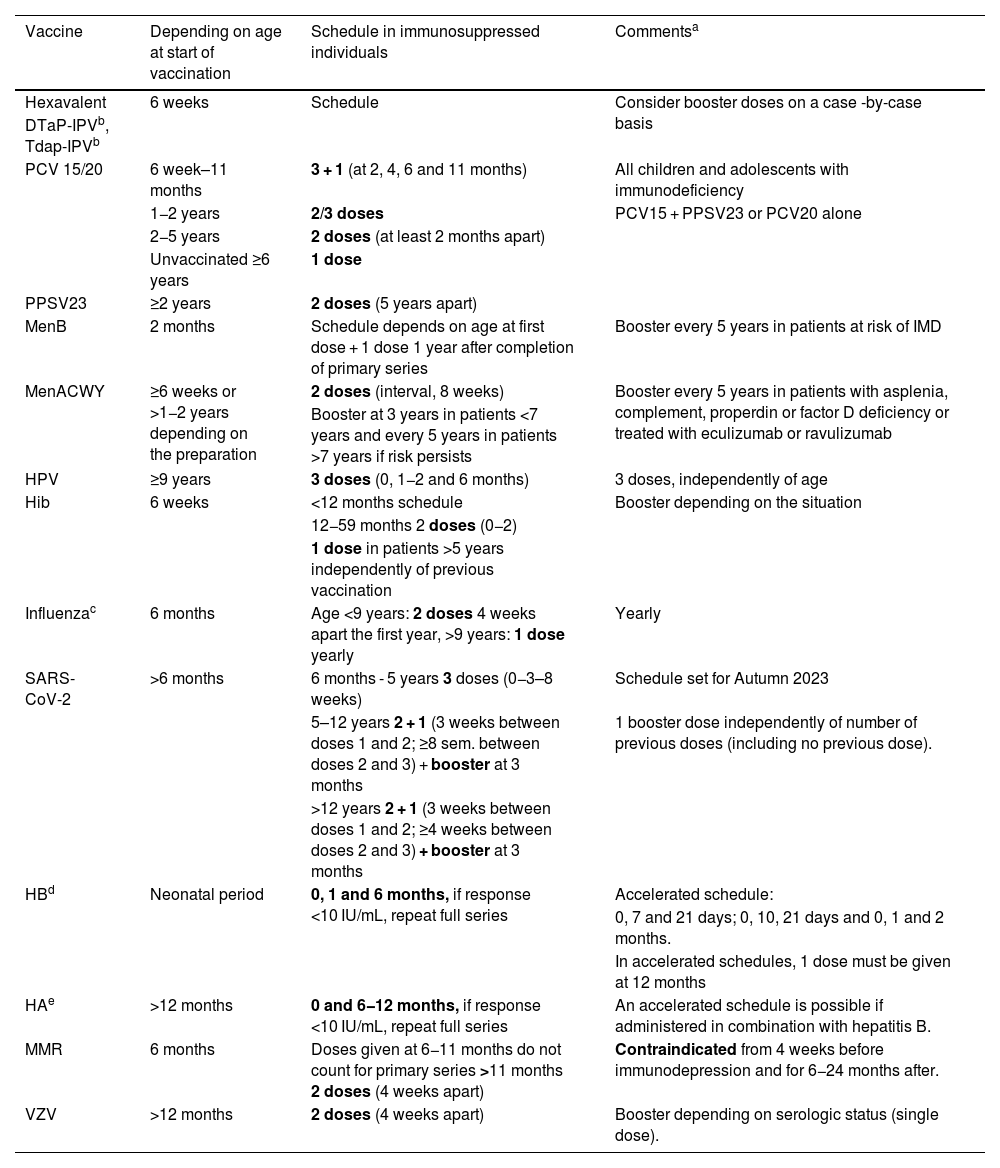
General recommendations for vaccination of immunosuppressed children and adolescents.
| Vaccine | Depending on age at start of vaccination | Schedule in immunosuppressed individuals | Commentsa |
|---|---|---|---|
| Hexavalent | 6 weeks | Schedule | Consider booster doses on a case -by-case basis |
| DTaP-IPVb, Tdap-IPVb | |||
| PCV 15/20 | 6 week–11 months | 3 + 1 (at 2, 4, 6 and 11 months) | All children and adolescents with immunodeficiency |
| 1−2 years | 2/3 doses | PCV15 + PPSV23 or PCV20 alone | |
| 2−5 years | 2 doses (at least 2 months apart) | ||
| Unvaccinated ≥6 years | 1 dose | ||
| PPSV23 | ≥2 years | 2 doses (5 years apart) | |
| MenB | 2 months | Schedule depends on age at first dose + 1 dose 1 year after completion of primary series | Booster every 5 years in patients at risk of IMD |
| MenACWY | ≥6 weeks or >1−2 years depending on the preparation | 2 doses (interval, 8 weeks) | Booster every 5 years in patients with asplenia, complement, properdin or factor D deficiency or treated with eculizumab or ravulizumab |
| Booster at 3 years in patients <7 years and every 5 years in patients >7 years if risk persists | |||
| HPV | ≥9 years | 3 doses (0, 1−2 and 6 months) | 3 doses, independently of age |
| Hib | 6 weeks | <12 months schedule | Booster depending on the situation |
| 12−59 months 2 doses (0−2) | |||
| 1 dose in patients >5 years independently of previous vaccination | |||
| Influenzac | 6 months | Age <9 years: 2 doses 4 weeks apart the first year, >9 years: 1 dose yearly | Yearly |
| SARS-CoV-2 | >6 months | 6 months - 5 years 3 doses (0−3–8 weeks) | Schedule set for Autumn 2023 |
| 5–12 years 2 + 1 (3 weeks between doses 1 and 2; ≥8 sem. between doses 2 and 3) + booster at 3 months | 1 booster dose independently of number of previous doses (including no previous dose). | ||
| >12 years 2 + 1 (3 weeks between doses 1 and 2; ≥4 weeks between doses 2 and 3) + booster at 3 months | |||
| HBd | Neonatal period | 0, 1 and 6 months, if response <10 IU/mL, repeat full series | Accelerated schedule: |
| 0, 7 and 21 days; 0, 10, 21 days and 0, 1 and 2 months. | |||
| In accelerated schedules, 1 dose must be given at 12 months | |||
| HAe | >12 months | 0 and 6−12 months, if response <10 IU/mL, repeat full series | An accelerated schedule is possible if administered in combination with hepatitis B. |
| MMR | 6 months | Doses given at 6−11 months do not count for primary series >11 months 2 doses (4 weeks apart) | Contraindicated from 4 weeks before immunodepression and for 6−24 months after. |
| VZV | >12 months | 2 doses (4 weeks apart) | Booster depending on serologic status (single dose). |
IMD, invasive meningococcal disease.
Inactivated vaccines should be given at least 2 weeks before initiation of immunosuppressive therapy and 3−6 months after its completion. Attenuated vaccines should be given at least 4 weeks before initiation of immunosuppressive therapy and 3–24 months after its completion and are contraindicated during treatment. Besides the booster doses generally recommended for immunosuppressed patients, necessary booster doses vary depending on the risk group.
DTaP: the full-dose toxoid vaccine is recommended by some authors regardless of age in immunosuppressed patients, such as HSCT recipients. Hexavalent vaccines are authorised for catch-up vaccination following immunosuppressive therapy independently of age.
Influenza: consider additional doses in situations in which the circulating strain is very different from those of previous seasons, independently of vaccination status. The intranasal live attenuated influenza vaccine is contraindicated.
HB. In patients with a poor response (anti-HB antibodies ≤10 mUI/mL), revaccinate with another 3 doses (at 0, 1 and 6 months). In patient >15 years, consider using the high-dose or adjuvanted vaccine. If anti-HB antibodies remain ≤10 mUI/mL after revaccination, the patient is considered a non-respondent and should receive specific immunoglobulin in the case of exposure.
HA. Revaccinate with 2 doses if the IgG level < 10 mIU/mL after the HA primary vaccination series. Antibody levels should be measured 1–2 months after, and if they are poor (<10 mIU/mL), additional vaccination is not recommended, and education should be provided on how to avoid infection, including the need to receive polyvalent immunoglobulins after exposure.
Individuals vaccinated at intervals other than those recommended or during treatment should be considered as potential nonresponders and the need for a booster dose assessed, performing serologic tests serving as correlates of protection, when available, a month after the administration of the most recent dose to guide revaccination, and considering the use of passive immunoprophylaxis and/or chemoprophylaxis in the case of exposure of individuals at risk.
Caregivers and household members should be fully and correctly vaccinated, with particular emphasis on the MMR, varicella, seasonal influenza and SARS-CoV-2 vaccines. The oral vaccines against poliovirus and Salmonella typhi Ty21 are contraindicated. The intranasal live attenuated influenza vaccine can be administered to the close contacts of immunosuppressed patients with the exception of those who have undergone HSCT in the past 2 months, with graft versus host disease (GvHD) or with severe combined immunodeficiency (SCID).
As a precaution, immunosuppressed individuals should avoid contact with the faecal material of an infant vaccinated against rotavirus for 4 weeks following vaccination and, in the case of close contacts who develop a rash after vaccination against varicella, avoid contact with the lesions.
Vaccination in paediatric haematopoietic stem cell and solid organ transplant recipientsHaematopoietic stem cell transplant recipients (Table 3)8–12Paediatric HSCT recipients experience immunosuppression to a degree that depends on the underlying disease and type of transplant, and is most severe and lasting in the case of allogeneic transplants. The changes in immunity result in decreased vaccine immunogenicity, so some time must be allowed to pass after transplantation to implement an adapted immunization schedule to achieve complete vaccination.
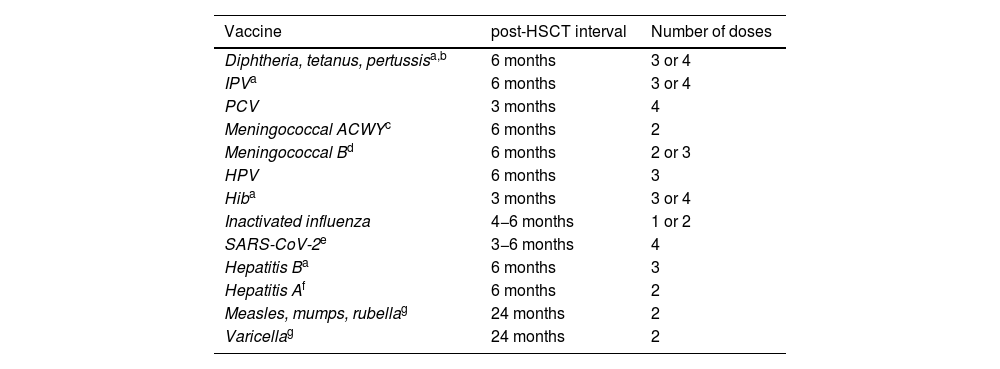
Vaccines, interval to vaccination following HSCT and number of doses needed.
| Vaccine | post-HSCT interval | Number of doses |
|---|---|---|
| Diphtheria, tetanus, pertussisa,b | 6 months | 3 or 4 |
| IPVa | 6 months | 3 or 4 |
| PCV | 3 months | 4 |
| Meningococcal ACWYc | 6 months | 2 |
| Meningococcal Bd | 6 months | 2 or 3 |
| HPV | 6 months | 3 |
| Hiba | 3 months | 3 or 4 |
| Inactivated influenza | 4−6 months | 1 or 2 |
| SARS-CoV-2e | 3−6 months | 4 |
| Hepatitis Ba | 6 months | 3 |
| Hepatitis Af | 6 months | 2 |
| Measles, mumps, rubellag | 24 months | 2 |
| Varicellag | 24 months | 2 |
Hexavalent vaccines can be used at any age to give fewer shots, always with the prior consent of the family. Doses should be given at least 1 month apart.
Vaccination against pneumococcal disease should start from 3 months from HSC transplantation with conjugate vaccines, administering 3 doses at least 1 month apart and a fourth dose 6 months after the third. In addition, the remaining conjugate vaccines should be administered starting from 6 months post transplantation, including the tetanus toxoid and reduced diphtheria toxoid and acellular pertussis (Tdap), inactivated poliovirus (IPV), hepatitis B (HB), Haemophilus influenzae type b (Hib), meningococcal B/ACWY and influenza vaccines. During the flu season, the influenza vaccine can be administered from 4 months post-HSCT, and if the patient has GvHD, a booster can be given 4–8 weeks later. Immunization against human papillomavirus with 9-valent vaccine (HPV9, 3 doses) is recommended starting at age 9 years.
The MMR and varicella vaccines can be administered from 24 months (2 doses at least 1 month apart) as long as the patient does not have GvHD, is not receiving immunosuppressant drugs, has not received immunoglobulin replacement therapy in the past few months and the CD4 count is greater than 200/mm3.
Solid organ transplant recipients8,9,11,12It is essential to vaccinate the patient as fully as possible before solid organ transplantation, even by implementing an accelerated schedule. After transplantation, the recipient will be immunosuppressed so the response to inactivated vaccines will not be optimal and live attenuated vaccines will be contraindicated.
Vaccination before solid organ transplantation should stop at least 4 weeks prior in the case of live vaccines and 2 weeks prior in the case of inactivated vaccines. Vaccination can resume 6 months after transplantation.
Before transplantationThe MMR and varicella vaccines can be administered earlier than scheduled starting from age 6 months. Administration of the HB and hepatitis A (HA) vaccines is indicated, and assessment of immunity after vaccination is recommended (Table 2, notes 3 and 4). Ensuring correct vaccination against pneumococcal disease is essential. Vaccination against huma papillomavirus with 3 doses of HPV9 is indicated for any age.
After transplantation (Table 4)Live vaccines (MMR, varicella, rotavirus, yellow fever, oral typhoid fever) are contraindicated. In the case of inactivated vaccines, even if the patient is fully vaccinated, it is recommended to administer booster doses of IPV IPV, DTaP/Tdap, pneumococcal vaccine and SARS-CoV-2 vaccine. Administration of the influenza vaccine is recommended from 1 month post transplantation. The patient should be given the pneumococcal vaccine with the highest valency available. Performance of hepatitis A and B immunity tests is recommended after post-SOT (see Table 2) and every 2 years thereafter, administering a booster dose if the test result is negative.
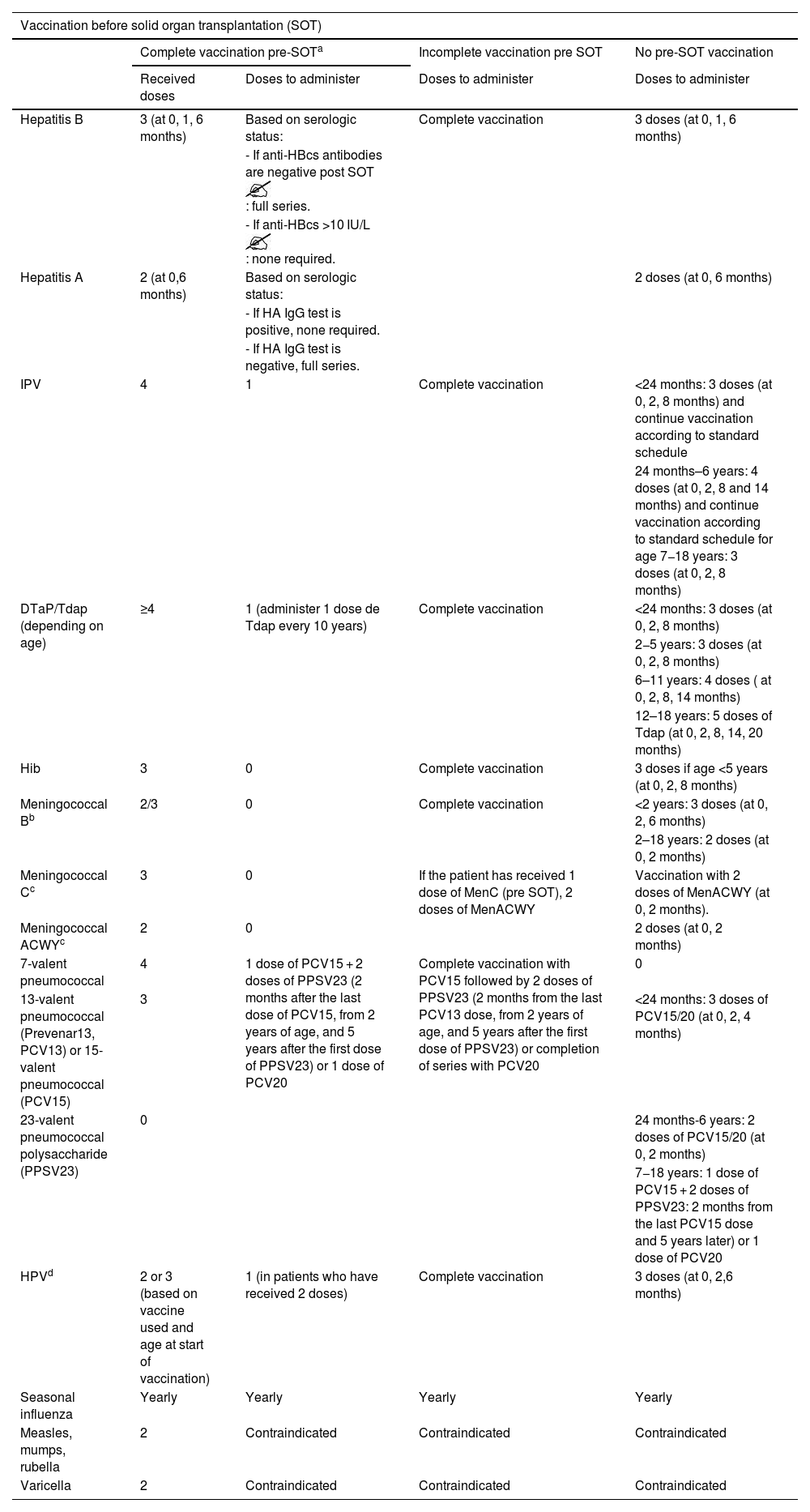
Vaccines, required number of doses and intervals based on complete or incomplete vaccination status before solid organ transplantation.
| Vaccination before solid organ transplantation (SOT) | ||||
|---|---|---|---|---|
| Complete vaccination pre-SOTa | Incomplete vaccination pre SOT | No pre-SOT vaccination | ||
| Received doses | Doses to administer | Doses to administer | Doses to administer | |
| Hepatitis B | 3 (at 0, 1, 6 months) | Based on serologic status: | Complete vaccination | 3 doses (at 0, 1, 6 months) |
| - If anti-HBcs antibodies are negative post SOT : full series. | ||||
| - If anti-HBcs >10 IU/L : none required. | ||||
| Hepatitis A | 2 (at 0,6 months) | Based on serologic status: | 2 doses (at 0, 6 months) | |
| - If HA IgG test is positive, none required. | ||||
| - If HA IgG test is negative, full series. | ||||
| IPV | 4 | 1 | Complete vaccination | <24 months: 3 doses (at 0, 2, 8 months) and continue vaccination according to standard schedule |
| 24 months–6 years: 4 doses (at 0, 2, 8 and 14 months) and continue vaccination according to standard schedule for age 7−18 years: 3 doses (at 0, 2, 8 months) | ||||
| DTaP/Tdap (depending on age) | ≥4 | 1 (administer 1 dose de Tdap every 10 years) | Complete vaccination | <24 months: 3 doses (at 0, 2, 8 months) |
| 2−5 years: 3 doses (at 0, 2, 8 months) | ||||
| 6–11 years: 4 doses ( at 0, 2, 8, 14 months) | ||||
| 12–18 years: 5 doses of Tdap (at 0, 2, 8, 14, 20 months) | ||||
| Hib | 3 | 0 | Complete vaccination | 3 doses if age <5 years (at 0, 2, 8 months) |
| Meningococcal Bb | 2/3 | 0 | Complete vaccination | <2 years: 3 doses (at 0, 2, 6 months) |
| 2–18 years: 2 doses (at 0, 2 months) | ||||
| Meningococcal Cc | 3 | 0 | If the patient has received 1 dose of MenC (pre SOT), 2 doses of MenACWY | Vaccination with 2 doses of MenACWY (at 0, 2 months). |
| Meningococcal ACWYc | 2 | 0 | 2 doses (at 0, 2 months) | |
| 7-valent pneumococcal | 4 | 1 dose of PCV15 + 2 doses of PPSV23 (2 months after the last dose of PCV15, from 2 years of age, and 5 years after the first dose of PPSV23) or 1 dose of PCV20 | Complete vaccination with PCV15 followed by 2 doses of PPSV23 (2 months from the last PCV13 dose, from 2 years of age, and 5 years after the first dose of PPSV23) or completion of series with PCV20 | 0 |
| 13-valent pneumococcal (Prevenar13, PCV13) or 15-valent pneumococcal (PCV15) | 3 | <24 months: 3 doses of PCV15/20 (at 0, 2, 4 months) | ||
| 23-valent pneumococcal polysaccharide (PPSV23) | 0 | 24 months-6 years: 2 doses of PCV15/20 (at 0, 2 months) | ||
| 7−18 years: 1 dose of PCV15 + 2 doses of PPSV23: 2 months from the last PCV15 dose and 5 years later) or 1 dose of PCV20 | ||||
| HPVd | 2 or 3 (based on vaccine used and age at start of vaccination) | 1 (in patients who have received 2 doses) | Complete vaccination | 3 doses (at 0, 2,6 months) |
| Seasonal influenza | Yearly | Yearly | Yearly | Yearly |
| Measles, mumps, rubella | 2 | Contraindicated | Contraindicated | Contraindicated |
| Varicella | 2 | Contraindicated | Contraindicated | Contraindicated |
Vaccination against meningococcal B disease is considered complete with a 3-dose series if primary vaccination was initiated before age 24 months, as long as the last dose was administered 12 months after, and considered complete with 2 doses if the primary series started after age 24 months.
Inborn errors of immunity (IEIs) are a heterogeneous group of diseases associated with a growing number of gene variants13,14 and with a variable risk of infection, so vaccination must be individualised in affected patients. For practical purposes, IEIs are classified into 3 groups: defects in adaptive immunity, defects in innate immunity and IEIs associated with characteristic phenotypes or syndromic immunodeficiencies.15
Inborn errors of immunity carry an increased risk of vaccine-preventable diseases and a reduced immune response, both in duration and intensity. Nevertheless, there is evidence of the protective effect of vaccination, associated with a decrease in mortality and the incidence of complications,15 which is why these patients usually require booster doses.
Live attenuated vaccines (viral or bacterial) carry a risk of dissemination in patients with defects in cell-mediated immunity,15,16 and while suspicion of an IEI is not sufficient reason to delay vaccination, live attenuated vaccines are contraindicated until the level of immune impairment is established.16
Inactivated or subunit vaccines are safe and can be effective. In patients with IEIs requiring immunoglobulin replacement therapy, live attenuated viral vaccines should not be administered for the next 3–11 months due to the interference of the treatment with the vaccine response.
Considering the above, children IEIs generally require an expanded vaccination schedule,17,18 especially for protection against encapsulated bacteria: pneumococcus, meningococcus (B/ACWY) and Hib, and from 6 months, annual vaccination against influenza with inactivated quadrivalent vaccine and against SARS-CoV-2 in adherence with current recommendations.18 It is also essential to promote the vaccination of household members and the general population.19
Table 5 presents an approximate vaccination schedule for the most frequent IEIs.
Vaccination in the most frequent inborn errors of immunity.
| Innate error of immunity | Vaccines that are contraindicated or should not be administered | Indicated vaccines | Comments |
|---|---|---|---|
| Cellular immunodeficiencies | All live attenuated vaccines | Expanded vaccination against encapsulated bacteria (pneumococcus, meningococcus [B/ACWY] and Hib), in addition to the SARS-COV-2 vaccine and the annual inactivated influenza vaccine | Most guidelines recommend administration of all inactivated vaccines included in the routine vaccination schedule, while others do not recommend it (with the exception of the influenza vaccine) if the patient is receiving treatment with immune globulins |
| Major antibody deficiency (pure B cell disorders): X-linked agammaglobulinaemia, common variable immunodeficiency, activated PI3K delta syndrome (APDS) | All live vaccines | Expanded vaccination against encapsulated bacteria (meningococcus and pneumococcusa), and vaccination against SARS-COV-2 and annual vaccination against influenza with inactivated vaccine Other inactivated vaccines in the routine schedule | The rotavirus vaccine can be given The MMR and varicella vaccines are not necessary if the patient receives immune globulins, as they would provide passive immunization against these infections and could interfere with the immune response to the vaccine. |
| Minor antibody deficiency: IgA deficiency, IgG subclass deficiency | Oral poliovirus vaccine | All vaccines in the routine schedule, including the MMR and varicella vaccines. Annual vaccination against influenza | If the patient has frequent respiratory symptoms, administration of the pneumococcal conjugate vaccine is recommended (PCV15 or 20). |
| Combined T and B cell or syndromic immunodeficiencies: Di George syndrome, ataxia-telangiectasia, Wiskott–Aldrich syndrome, hyper-IgE syndrome, hyper-IgM syndrome, chronic mucocutaneous candidiasis | Expanded vaccination against encapsulated bacteria (pneumococcus, meningococcus [B/ACWY] and Hib), in addition to the SARS-COV-2 vaccine and the annual inactivated influenza vaccine | May receive live attenuated viral vaccines if CD4 count ≥500 cells/mm3 (>1000 in children aged 1−6 years; 1500 in infants <1 year) and CD8 count ≥200 cells/mm3 | |
| Complement deficiencies: late common pathway, properdin or factor D deficiency | None | All vaccines in the routine schedule, including the MMR and varicella vaccines. | Vaccination with 1 dose of Hib is recommended in previously unvaccinated children aged >5 years. |
| Must receive expanded vaccination against encapsulated bacteria (pneumococcus meningococcus [B/ACWY, considering revaccination every 3−5 years] and Hib), in addition to the SARS-COV-2 vaccine and the annual inactivated influenza vaccine | Consider other measures, such as antibiotic prophylaxis | ||
| Disorders of phagocyte number or function: chronic granulomatous disease (CGD), adhesion molecule defects such as leukocyte adhesion deficiency (LAD), Chediak–Higashi syndrome, congenital neutropenia | Live bacterial vaccines (BCG and oral attenuated typhoid fever vaccine). Live viral vaccines are contraindicated in LAD and S. de Chediak–Higashi, but not in CGD or congenital neutropenia (5) | All vaccines in the routine schedule save for the MMR and varicella vaccines in patients with LAD or Chediak–Higashi syndrome, in whom they are contraindicated. Vaccination against influenza is a must, as influenza increases the risk of infection by S. aureus | Vaccination against pneumococcal disease with con PCV15 + PPSV23 or PCV20 alone |
| Innate immune defects: (defects of the IL-12 axis/interferon gamma) | All live attenuated vaccines, bacterial (BCG and oral attenuated typhoid fever vaccine) or viral (MMR and varicella) | All vaccines in the routine schedule save for live attenuated vaccines | Vaccination against pneumococcal disease with con PCV15 + PPSV23 or PCV20 alone |
| Innate immune defects: (IRAK4 and MyD88 deficiency) | None | All vaccines in the routine schedule. Vaccination against pneumococcal disease with con PCV15 + PPSV23 or PCV20 alone, and vaccination against SARS-COV-2 and annual vaccination against influenza |
Expanded vaccination against encapsulated bacteria: Haemophilus influenzae type b, pneumococcus and all meningococcal serogroups (B/ACWY). At the start of immunization in the early months of life, the patient should always be vaccinated with pneumococcal conjugate vaccine in a 3 + 1 schedule, with the additional use of PPSV23 in children aged more than 2 years, if the PCV15 was given, and at least 8 weeks apart from the last administered dose, or else administering the PCV20.17
Immunosuppression in oncological paediatric patients persists for months after completion of treatment, affecting the immunogenicity of vaccines administered before, during or soon after chemotherapy.8,20,21 In addition, some patients may have anatomic or functional asplenia (radiation therapy to the abdomen, GvHD). The evidence regarding vaccination in the context of novel immunotherapies is still insufficient.20,21
General principles for vaccination of children managed with chemotherapyBefore chemotherapyIf feasible, getting vaccination up to date according to the routine immunization schedule is recommended, administering inactivated vaccines at least 2 weeks before and attenuated vaccines at least 4 weeks before initiation of chemotherapy.8
During chemotherapyVaccines in the routine immunization schedule should not be administered during intensive chemotherapy. The response to inactivated vaccines is very small (vaccination would be considered to have failed) and attenuated vaccines are contraindicated.
In children receiving maintenance treatment for lymphoblastic leukaemia, annual vaccination against influenza is recommended from age 6 months. The guidelines of the European Conference on Infections in Leukaemia also recommend a PCV booster dose,21 although the immune response may be suboptimal and administration of another dose is necessary after treatment has been completed.22,23
After chemotherapyRepeating primary vaccination with vaccines the child has already received is not necessary. In fully vaccinated children, administration of a booster dose of previously received vaccines is recommended, after which vaccines should be administered according to the routine immunization schedule based on the age of the patient.8,21 If the vaccination was not up to date at initiation of chemotherapy, catch-up vaccination will be the first step. The schedule for catch-up vaccination, including the number of doses of each vaccine, the minimum age for administration and the minimum intervals between doses, can be consulted in the tables published by the Advisory Committee on Vaccines of the AEP (CAV-AEP).24
Inactivated vaccines should be administered between 3 and (preferably) 6 months after completion of chemotherapy, while the administration of attenuated vaccines should be deferred to 6–12 months after (preferably 12). In regimes that include anti-B cell antibodies, an interval of 6–12 months is recommended before administration of any vaccine.8,21 Recent data show that children with lymphoblastic leukaemia could receive the pneumococcal conjugate vaccine right after completing treatment, as the response is similar to that of those vaccinated 6 months after.22 Early administration of this vaccine could be contemplated, especially in children who missed vaccination during maintenance chemotherapy.
Specific recommendations for vaccination of oncological patients- –
Pneumococcal: PCV15 + PCV23 sequence, or PCV20 exclusively.8
- -
Influenza: annual inactivated vaccines, at least the 3 seasons following completion of chemotherapy.8
- -
SARS-CoV-2: schedule depending on age and type of vaccine.18
- -
HPV9: recommended from age 9 years.18
- -
The use of live attenuated vaccines is not recommended in patients treated with tyrosine-kinase inhibitors or ruxolitinib.19
The earliest recommendations of the European Alliance of Associations for Rheumatology (EULAR) were developed in 2011, and many were extrapolated from the adult literature due to the low quality of the studies conducted in children. Since then, the evidence on the safety and immunogenicity of vaccines has doubled.
General recommendations- -
If possible, vaccines should be administered 2–4 weeks before initiation of immunosuppressive therapy (especially in the case of B-cell depleting therapies), but necessary treatment should never be deferred. However, the interval may vary depending on the type of drug (see Table 14.3 of the CAV-AEP at https://vacunasaep.org/documentos/manual/cap-14#7)
- -
Vaccination during the inactive phase of disease/remission is advisable, and it should not be delayed out of fear of triggering an exacerbation, as there is no evidence of such an association.
- -
All immunosuppressed patients must adhere to the routine vaccination schedule and travel vaccination requirements, with the exception of live attenuated vaccines.
- -
Inactivated vaccines can be administered to patients receiving corticosteroids or disease-modifying antirheumatic drugs (DMARDs).
- -
Live attenuated viral vaccines are contraindicated in patients with treatments resulting in high-level immunosuppression (Table 1), with the exception of MMR booster doses and the varicella vaccine, whose administration can be contemplated on a case-by-case basis under specific circumstances.
- -
Vaccination against tetanus according to current general recommendations; passive immunization is recommended for patients who have received B cell-depleting therapy in the past 6 months in whom administration of tetanus toxoid is indicated.
- -
Administration of HPV9 should be considered in previously unvaccinated patients with systemic lupus erythematosus or inflammatory bowel disease (3 doses if the patient receives immunosuppressive therapy).
- -
Administration of the MMR booster dose could be considered on a case-by-case basis, after assessment by the specialist in charge, in patients receiving methotrexate (MTX) at immunomodulating doses (A-level recommendation) or low-dose steroids, anti-TNFα, anti-IL-1 or anti-IL-6 (C-level recommendation).
- -
Administration of the varicella vaccine could be considered on a case-by-case basis, after assessment by the specialist in charge, in naïve patients receiving methotrexate (MTX) at immunomodulating doses A-level recommendation) or low-dose steroids, anti-TNFα, anti-IL-1 or anti-IL-6 (D-level recommendation).
- -
Vaccination against yellow fever should be avoided in any patient receiving immunosuppressive therapy (D-level recommendation)
- -
Vaccination against hepatitis A is recommended in patients treated with hepatotoxic immunosuppressive drugs (MTX or tocilizumab).
Vaccination against hepatitis B is essential, in addition to monitoring of the vaccine response. Vaccination against hepatitis A is also indicated, with the standard schedule or alternatively with the combination vaccine (A + B) if a booster dose of HB is required. In the absence of immunosuppression, vaccination against measles and varicella is recommended as soon as possible after diagnosis.
Cardiovascular and respiratory diseases25,26This includes children with congenital heart diseases (cyanotic diseases, associated with heart failure or haemodynamic changes), pulmonary hypertension, bronchopulmonary dysplasia, cystic fibrosis, bronchiectasis and moderate to severe asthma. Vaccination against varicella according to the routine schedule is recommended, with the qualification that in patients treated with salicylates it is considered preferable to discontinue treatment for 6 weeks after the administration of the vaccine (if not possible, the patient should still be vaccinated, as there have been no reports of Reye syndrome associated with the vaccine-type virus).
Neurologic diseases25,26No vaccines are contraindicated for children with epilepsy. In fact, the use of the vaccine with acellular pertussis has been found to significantly decrease the incidence of febrile seizures associated with the diphtheria, tetanus and whole-cell pertussis vaccines. If the patient receives hepatotoxic drugs, such as valproic acid, vaccination against hepatitis A is recommended.
However, in the presence of a progressive, unstable or unspecified encephalopathy, the administration of vaccines that could destabilize the patient (like the pertussis vaccine) should be deferred. In the case of neurological autoimmune or inflammatory disease in which there are grounds to suspect an association with a given vaccine, no other doses of the vaccine should be administered.
Genetic disorders (Down syndrome)25,26Annual vaccination against influenza and vaccination against pneumococcal disease (with PCV15 + PPSV23 or PCV20 exclusively) and hepatitis A is recommended for children with Down syndrome.
For children with other genetic disorders, specific vaccination requirements need only be assessed if there are chronic immune comorbidities.
Haemoglobin disorders25,26Children with haemoglobin disorders manifesting with functional hyposplenism must be vaccinated against pneumococcal disease (with PCV15 + PPSV23 or PCV20), Hib and meningococcal disease (Men ACWY + booster dose at 5 years and Men B with booster doses 1 year after completion of the primary series and every 5 years thereafter), and also against hepatitis (A and B) due to the increased risk of liver disease. It is important to consider the recommended intervals (6–7 months) between the transfusion of blood products and the administration of attenuated vaccines. In Spain, as long as there is no outbreak of measles, mumps or rubella, changes to the immunization schedule are not indicated if the patient is going to be treated with hydroxyurea in the first year of life.
Patients with beta thalassaemia minor or sickle cell trait should receive the same vaccines as the general population.
Cochlear implant25,26Due to the risk of developing meningitis and otitis media, vaccination against pneumococcal disease is particularly recommended (with PCV15 + PPSV23 or PCV20), in addition to Hib and annual vaccination against influenza.
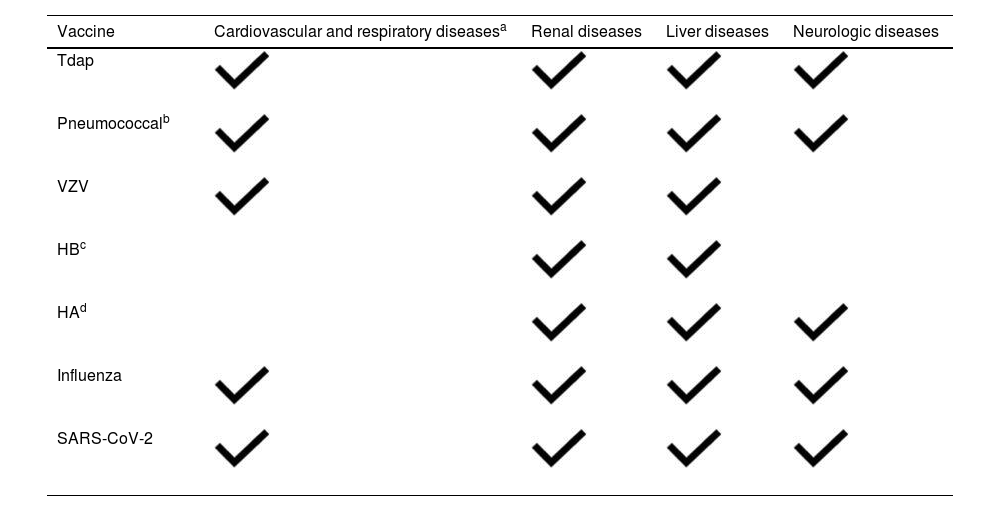
Table 6 presents an approximate vaccination schedule for immunosuppressed children with chronic diseases.
Vaccines specifically recommended for different chronic diseases.
| Vaccine | Cardiovascular and respiratory diseasesa | Renal diseases | Liver diseases | Neurologic diseases |
|---|---|---|---|---|
| Tdap | ||||
| Pneumococcalb | ||||
| VZV | ||||
| HBc | ||||
| HAd | ||||
| Influenza | ||||
| SARS-CoV-2 |
HA, hepatitis A; vaccine HB, hepatitis B vaccine; HPV, human papillomavirus vaccine; Tdap, reduced toxoid tetanus, diphtheria and acellular pertussis vaccine; VZV, varicella zoster vaccine.
Including children with cyanotic congenital heart disease, heart disease manifesting with heart failure or haemodynamic instability, pulmonary hypertension, bronchopulmonary dysplasia, cystic fibrosis, bronchiectasis and asthma with a high risk of exacerbation.
Conjugated vaccine (PCV20 or PCV15) followed by 1 dose of PPSV23 and a booster at 5 years in children aged >2 years at high risk, such as children with asplenia, a cochlear implant, loss of cerebrospinal fluid, heart disease, diabetes, liver disease, chronic pulmonary disease or receiving immunosuppressive treatment.
Monitoring the vaccine response is particularly important in patients with liver disease. If the post-vaccine level of anti-HB is <10 mIU/mL, the full series should be administered again (0, 1 and 6 months); if the antibody screen continues to be negative, the patient should be considered a non-respondent, and no further doses should be given.
Before traveling, immunosuppressed children should have their vaccines up to date, and it is a good time to administer upcoming doses, possibly earlier than established in the vaccination schedule, if their immune status allows it.32–39 Individualised guidance must be provided sufficiently in advance to ensure that administered vaccines are effective. Thus, it is recommended that the child make a visit between 4 and 6 weeks prior to the departure. If it can be avoided, immunosuppressed children aged less than 2 years should not travel.
Specific vaccination of travellers32–39Routine/universal vaccinesThose recommended in the routine vaccination schedule for children of the traveller’s age. If the child’s vaccination is not up to date, the missing doses will be administered before travel. If the child is to travel to a country endemic for a disease against which the child has not been vaccinated, such as measles or varicella, the vaccine will be administered earlier than scheduled as long as it is possible.
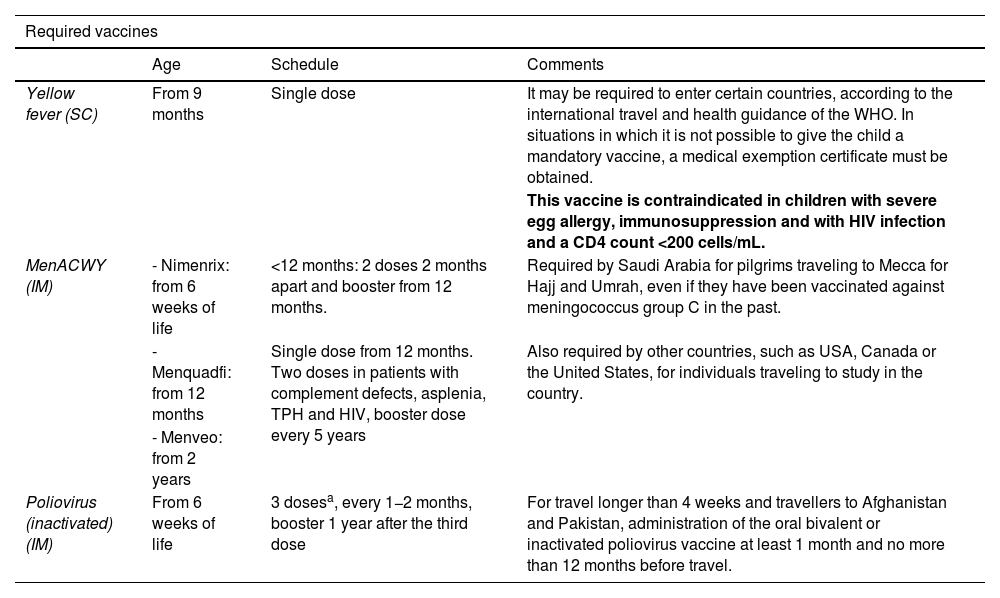
Specific vaccines for travellers (see Table 7)- •
Mandatory: depending on the destination, type of travel and age. It includes those required by receiving countries for entry: yellow fever, meningococcal and poliovirus. The authorities of countries with specific requirements will expect travellers to carry an International Certificate of Vaccination or Prophylaxis correctly recording the received vaccinations.
- •
Recommended depending on travel: depending on the destination, the risk assessment of the child and the type of travel.
Table 7.Specific vaccines for travellers: required and recommended depending on the travel plan.
Required vaccines Age Schedule Comments Yellow fever (SC) From 9 months Single dose It may be required to enter certain countries, according to the international travel and health guidance of the WHO. In situations in which it is not possible to give the child a mandatory vaccine, a medical exemption certificate must be obtained. This vaccine is contraindicated in children with severe egg allergy, immunosuppression and with HIV infection and a CD4 count <200 cells/mL. MenACWY (IM) - Nimenrix: from 6 weeks of life <12 months: 2 doses 2 months apart and booster from 12 months. Required by Saudi Arabia for pilgrims traveling to Mecca for Hajj and Umrah, even if they have been vaccinated against meningococcus group C in the past. - Menquadfi: from 12 months Single dose from 12 months. Two doses in patients with complement defects, asplenia, TPH and HIV, booster dose every 5 years Also required by other countries, such as USA, Canada or the United States, for individuals traveling to study in the country. - Menveo: from 2 years Poliovirus (inactivated) (IM) From 6 weeks of life 3 dosesa, every 1−2 months, booster 1 year after the third dose For travel longer than 4 weeks and travellers to Afghanistan and Pakistan, administration of the oral bivalent or inactivated poliovirus vaccine at least 1 month and no more than 12 months before travel. Recommended vaccines Cholera (VO) From 2 years A) Inactivated. 2−3 doses. Exceptional indication for travellers to areas with current outbreaks or highly endemic, such as Benin, Burundi, Cameroon, Democratic Republic of the Congo, Ethiopia, Kenia, Malawi, Mozambique, Nigeria, Somalia, Sudan, Uganda, Bangladesh, India, Yemen, Haiti and Philippines 2−6 years: 3 doses. Booster at 6 months >6 years: 2 doses. Booster at 2 years B) Attenuated. 1 dose. 2−6 years: 1 dose de 50 mL >6 years: 1 dose de 100 mL Central European encephalitis (IM) From 12 months 3 doses (0, 1−3 months and 6−15 months). Booster every 5 years Travellers to risk areas with stays longer than 3−4 weeks in wooded areas of Russia and Northeastern and Central Europe (especially Lithuania, Czech Republic, Germany and Sweden). Accelerated schedule: 0, 7, 21 days, or else 0, 14 days Booster 12−18 months Japanese encephalitis (IM) From 2 months of age 2 doses at days 0 and 28 Travellers who are going to spend long periods out of doors (camping, hiking) in rural areas with endemic transmission during the rainy season, chiefly in Japan, Southeast Asia and the Western Pacific region Typhoid fever A) From 2 years of age A) Single dose. Booster every 2−3 years Travel to areas of North and West Africa, South Asia, Indonesia and Peru A) IM B) VO B) From 6 years (in summary of product characteristics, 3 years) B) 3 doses. 3 capsules taken in alternating days on an empty stomach. Revaccinate every 1−3 years if the risk persists Hepatitis A (IM) From 12 months 2 doses: 0 and 6−12 months In patients at risk, it can be given from 6 months, but the does not count toward the routine vaccine series Rabies (IM and ID) Do not give to children under 1 year for prevention, but give for prophylaxis if there is risk due to a bite De 2 a 6 doses Children who are to travel for prolonged stays to endemic areas, especially remote areas or going to caves, where treatment with immune globulin may be difficult to access Pre-exposure course: ID: 02, 72 and 282b, 4 or 6 doses IM: 0, 7 and 28b days, 2 or 3 doses Booster in 2−5 years Post-exposure course: (+Human rabies immune globulin) ID: 0, 3 and 7 at 2 sites, 6 doses IM: 0, 3, 7 and 21, 4 doses IM: 0 at 2 sites, 7 and 21, 4 doses Dengue (SC) From 4 years of age 2 doses: 0 and 3 months Contraindicated in patients with history of anaphylaxis to any component, immunodeficiencies, pregnant or breastfeeding women Tuberculosis (ID) From birth Single dose: 0.1 mL in infants >1 year and 0.05 mL in children <1 year Can be considered in previously unvaccinated individuals with a negative Mantoux test depending on the destination and nature of the trip. Contraindicated in children <2 years who are household contacts of a suspected or confirmed case of active tuberculosis, HIV + individual, infants born to mothers who received immunosuppressive biologic agents during pregnancy and/or individuals currently receiving or who have received treatments affecting immunity in the past 6 months (chemotherapy, radiotherapy or biologic agents). ID, intradermal; IM, intramuscular; O, oral; SC, subcutaneous.
Table 8 presents the specific recommendations for vaccination of immunosuppressed children who plan to travel.
Specific recommendations for vaccination of immunosuppressed children who are travelling.
| HIV with CD4 > 200/mm3a | HIV/AIDS, CD4 < 200/mm3b | Severe, non-HIV immunosuppressed statusc | Chronic diseased | |
|---|---|---|---|---|
| BCG | Contraindicated | Contraindicated | Contraindicated | X |
| MMR | With caution or recommended | Contraindicated or with caution | Contraindicated | X |
| Typhoid fever (VO) | Contraindicated | Contraindicated | Contraindicated | X |
| Typhoid fever (IM) | X | X | X | X |
| Yellow fever | With caution | Contraindicated | Contraindicated | X |
| Japanese encephalitis | X | X | X | X |
| Central European encephalitis | X | X | X | X |
| Rabies | X | X | X | X |
| Cholera | X | X | X | X |
| Hepatitis A | Recommended | Recommended | X | X |
| Meningococcal, PCV 15/20, PPSV23 | Recommended | Recommended | X | Recommended |
| Influenza | Recommended | Recommended | Recommended | Recommended |
X: same as vaccination recommended in healthy children.
HIV and CD4 > 200/mm3. Live attenuated vaccines should not be administered, with the exception of the MMR and varicella vaccines if the CD4 count >200. The response to inactivated vaccines may be suboptimal. Postponing travel (and therefore vaccination) for at least 3 months after immune reconstitution is recommended. Despite lower rates of seroconversion and antibody titres compared to healthy children, these patients respond with protective antibodies to most studied vaccines.
HIV/AIDS with CD4 < 200/mm3. In children with HIV, it is essential to know the CD4 count before travel. If the CD4 count is <200/mm3 and the patient is not receiving antiretroviral treatment, travel must be deferred until antiretroviral therapy is initiated and immune reconstitution achieved.
Severe non-HIV immunosuppression. Includes: patients with active leukaemia or lymphoma, generalised cancer, aplastic anaemia, graft-versus-host disease, inborn errors of immunity, current or very recent radiation therapy, first two years after solid organ or haematopoietic stem cell transplantation, or transplant recipients more than 2 years post transplantation, receiving immunosuppressive therapy.
Chronic diseases associated with limited immunodeficiency. Including: asplenia, chronic renal or liver disease, diabetes or complement deficiency. Asplenic patients are at greater risk of infection and disease by encapsulated bacteria, and while the vaccine response may be suboptimal, it is important to ensure they are correctly vaccinated against meningococcal disease (ABCWY), pneumococcal disease and Hib.
This research project did not receive specific financial support from funding agencies in the public, private or not-for-profit sectors.
Conflicts of interest (last 5 years)Irene Rivero Calle has collaborated in educational activities funded by GlaxoSmithKline, MSD, Pfizer and Sanofi Pasteur, as a researcher in vaccine clinical trials for Abbot, AstraZeneca, Enanta, Gilead, GlaxoSmithKline, HIPPRA, Janssen, Medimmune, Merck, Moderna, MSD, Novavax, Pfizer, Reviral, Roche, Sanofi and Seqirus; and as a consultant in GlaxoSmithKline, MSD, Pfizer and Sanofi advisory boards.
Teresa del Rosal has received fees for participation in conferences and from research grants from MSD, funding to attend educational activities from Pfizer and collaborated as researcher in clinical trials for GSK, Janssen and MSD, for which the institution she is affiliated with received fees.
Elisa Garrote has collaborated in educational activities funded by GlaxoSmithKline, MSD, Pfizer and Sanofi, as a researcher in vaccine clinical trials for de GlaxoSmithKline, and as a consultant in GlaxoSmithKline and MSD advisory boards.
Esmeralda Núñez has collaborated in educational activities funded by Pfizer.
María Luisa Navarro has collaborated in educational activities funded by Gilead, GlaxoSmithKline, Janssen, MSD, Pfizer and ViiV, as a consultant in Abbott, AstraZeneca, Novartis, Pfizer and ViiV advisory boards and in clinical trials funded by GlaxoSmithKline, Pfizer, Roche, MSD, Sanofi and Pfizer.
José Tomas has received fees for participation in conferences from Gilead, GSK, ViiF and Pfizer. He has received grants or funding to attend training workshops from BioMerieux, Gilead and Pfizer. He has received fees as a consultant for Pfizer, GSK and ViiF. He has collaborated as the principal investigator of the centre in clinical trials of Pfizer, GSK and Janssen, for which his institution collected fees.
Cristina Calvo has received fees for participation in conferences from MSD and Pfizer. She has received research grants/funding from MSD and BioMerieux. She has received fees as a consultant for Sanofi. She has participated as a researcher in clinical trials conducted by Abbott, MSD, Pfizer, Janssen, GSK and Allergan, for which her institution collected fees.
Francisco Álvarez García has received funding to attend domestic educational activities. He has participated in educational activities funded by Alter, AstraZeneca, GSK, Pfizer, MSD and Sanofi and served as a consultant in GSK, Pfizer, MSD and Sanofi advisory boards.
Francisco José Álvarez García (FJAG), Antonio Iofrío de Arce (AIA), Javier Álvarez Aldeán (JAA), María Garcés-Sánchez (MGS), Elisa Garrote Llanos (EGL), Abián Montesdeoca Melián (AMM), Marisa Navarro Gómez (MLNG), Valentín Pineda Solas (VPS), Irene Rivero Calle (IRC), Jesús Ruiz-Contreras (JRC) and Pepe Serrano Marchuet (PSM).