In the management of adolescent obesity, therapeutic options are limited and the outcomes of lifestyle modification (LM) alone are poor. Liraglutide, a GLP-1 receptor agonist, was the first drug approved in Spain for the management of obesity in adolescents aged 12 years or older.
ObjectiveTo evaluate the effectiveness of liraglutide combined with LM in adolescents with obesity compared to LM alone.
MethodsRetrospective observational study of 62 adolescents (12-18 years) with a body mass index (BMI) at or above the 95th percentile. The intervention group (n=31) received liraglutide plus LM, while controls (n=31) matched for age, sex and treatment duration were managed with LM alone. We analyzed anthropometric, cardiovascular and body composition variables at three time points: baseline (T1), end of treatment (T2: mean, 6.9 months; SD, 4.7), and follow-up (T3: mean, 12.5 months; SD, 4.9 months). Comparisons between groups were performed using adjusted analysis of covariance model for changes in quantitative variables and logistic regression for BMI reductions of 5% or greater and 10% or greater.
ResultsIn the intervention group, the BMI z score decreased significantly (mean, −1.09 [SD, 0.24] vs −0.10 [SD, 0.25] in controls; P=.001). This corresponded to a BMI reduction of 5% or greater in 48.4% of patients and 10% or greater in 29%, compared to 3% and 1%, respectively, in the control group (P<.05). The weight loss was maintained at six months of follow-up (T3). There was a significant reduction in insulin levels, the HOMA-IR, triglyceride levels, systolic hypertension (HTN), and the number of prediabetic patients.
ConclusionsLiraglutide combined with LM achieved a greater reduction in the BMI z score, waist/height ratio and cardiometabolic parameters compared to the LM alone. Further research is needed to assess its long-term effects and difficulties in its implementation.
La obesidad en adolescentes tiene opciones terapéuticas limitadas y los resultados con las modificaciones de estilos de vida (MEV) son escasos. Liraglutida, un agonista del receptor GLP-1, fue el primer fármaco aprobado en España para el manejo de la obesidad en ≥ 12 años.
ObjetivoEvaluar la efectividad de liraglutida combinado con MEV en adolescentes con obesidad, comparándolo con MEV exclusivamente.
MétodosEstudio observacional retrospectivo de 62 adolescentes (12-18 años) con IMC≥p95. El grupo intervención (n=31) recibió liraglutida junto con MEV, mientras el grupo control (n=31) emparejado por edad, sexo y tiempo en tratamiento, solo siguió MEV. Se analizaron variables antropométricas, cardiovasculares y de composición corporal en tres momentos: inicio (T1), fin del tratamiento (T2: 6,9±4,7 meses) y seguimiento (T3:12,5±4,9 meses). La comparación entre los dos grupos se realizó mediante un modelo ajustado de análisis de la covarianza para los cambios en las variables cuantitativas y regresión logística para la reducción del IMC≥5% y ≥ 10%.
ResultadosEn el grupo intervención, el Z-IMC disminuyó significativamente (-1,09±0,24 vs. -0,10±0,25 en controles, p=0,001). Esto supuso una pérdida de ≥ 5% del IMC en un 48,4% pacientes y ≥ 10% en un 29%, frente al 3% y 1% en el grupo control (p<0,05). La pérdida de peso se mantuvo tras seis meses de seguimiento (T3). Se observó además una reducción significativa de insulina, HOMA-IR, triglicéridos, hipertensión arterial (HTA) sistólica y número de pacientes prediabéticos.
Conclusiones. Liraglutida junto a MEV condujo a una mayor reducción del Z-IMC, del índice cintura/altura, y parámetros cardiometabólicos en comparación al grupo con sólo MEV. Es necesario investigar sus efectos a largo plazo y las dificultades para su implementación.
Historically, in the management of obesity in children and adolescents, treatment options have been limited and based on lifestyle modification (LSM) through diet and physical activity. This first-line treatment approach is not very effective in the short or long term, with particularly poor outcomes in patients with severe obesity and comorbidities.1,2 In 2007, in response to the growing prevalence of obesity, an expert committee formed by the American Academy of Pediatrics and another 14 medical societies of the United States published recommendations for the treatment of obesity in children and adolescents with a stepwise approach.3 If an adequate response was not achieved within 3 to 6 months of LSM, the committee proposed the addition of medication, but only in adolescents aged 12 years or older with severe forms of obesity, and only in tertiary care hospitals. In 2007, orlistat and sibutramine were the only drugs approved for this age group in the United States by the Food and Drug Administration (FDA), while none had been approved by the European Medicines Agency (EMA). In spite of these recommendations, the use of medication for pediatric obesity was never widely implemented, possibly because there were limited options and the efficacy of the available drugs was relatively low. In the 2010s, sibutramine was withdrawn from the market in the United States, leaving only orlistat, and some centers started to use drugs such as phentermine, topiramate and metformin off label, as LSM measures alone were insufficient to achieve significant and lasting weight loss. In addition, while bariatric surgery has increasingly become an accepted approach, it is neither widely accessible nor desired by many patients and their families. A decade later, the FDA (in 2020) and the EMA (in 2021) approved the use of liraglutide for treatment of obesity in adolescents aged 12 or more years.4,5
Liraglutide is a glucagon-like peptide-1 receptor agonist (GLP-1RA). Glucagon-like peptide-1 (GLP-1) receptors are present in the pancreas, intestine and hypothalamus, and their activation stimulates glucose-dependent insulin secretion, inhibits glucagon production, induces satiety by delaying gastric emptying and reduce appetite and intake through the activation and inhibition of neural pathways in the hypothalamus. The latter mechanism is most important for weight loss. The use of GLP-1RA was first approved for treatment of type 2 diabetes (T2D) in adults, but it quickly became apparent that, in addition to improving glycemic control, it promoted weight loss. This prompted performance of clinical trials in non-diabetic obese individuals, in whom it achieved greater weight loss compared to diabetic individuals, leading to its authorization for the treatment of obesity.6 At present, liraglutide (Saxenda®, Victoza®) is indicated for treatment of obesity in patients aged at least 12 years with a body mass index (BMI) at or above the 95th percentile (P95) and a body weight greater than 60kg. It is administered daily via the subcutaneous route and it is not funded by the Spanish public health system.
The aim of our study was to assess the effectiveness of liraglutide combined with LSM in a group of adolescents with obesity compared to a control group in which patients were only managed with LSM. We analysed anthropometric, cardiovascular and body composition variables.
Patients and methodsWe conducted a retrospective observational study in two groups of adolescent patients (12-18 years) with obesity (BMI≥P95) managed at the nutrition and metabolic disease unit of a tertiary care hospital between February 2022 and January 2024. We offered every patient who met the inclusion criteria the use of liraglutide as a pharmacotherapeutic measure in addition to LSM, and the family decided whether or not to use the drug, as it was not publicly funded. The liraglutide group included 31 patients who were treated for a minimum of 6 weeks. The initial dose was 0.6mg/day and was increased weekly based on the observed effectiveness and tolerance to up to a maximum of 3mg. We selected a control group (no liraglutide) of 31 patients matched for age, sex and date of treatment initiation in whom the managed consisted solely of LSM.
We analyzed changes in anthropometric variables (weight, height, waist circumference, waist-to-hip ratio and BMI z score [BMIz] using the Orbegozo tables7 as reference) at three timepoints: initiation of treatment with liraglutide (T1), completion of treatment (T2: mean, 6.9 months; SD, 4.7) and last visit after treatment completion (T3: mean, 12.5 months; SD, 4.9).
For T1 and T3, we also assessed changes in serum values following a 10-h fast (glucose, insulin, Homeostatic Model Assessment for Insulin Resistance [HOMA-IR], glycated hemoglobin [HbA1c], triglycerides, total cholesterol, high-density lipoprotein [HDL], low-density lipoprotein [LDL] and uric acid), blood pressure (BP), and body composition based on data obtained by means of bioelectrical impedance analysis (BIA) with the Akern BIA 101 Anniversary analyzer: resistance (Rz), reactance (Xc), phase angle (PA), fat-free mass (FFM) and fat mass (FM).
We defined systolic and/or diastolic hypertension (HTN) according to the clinical guidelines of the American Academy of Pediatrics.8 Prediabetes was defined based on two of the criteria proposed by the American Diabetes Association: a fasting glucose of 100 to 125mg/dL and/or an HbA1c concentration of 5.7% to 6.4%. The intensity of extracurricular physical activity was classified as low, moderate or vigorous based on the corresponding energy expenditure metabolic equivalent established in the study conducted by Ainsworth et al,9 and we documented the total hours of physical activity per week. The study was approved by the Ethics Committee of the hospital.
Statistical analysisWe summarized data for qualitative variables as absolute and relative frequency distributions. We expressed quantitative variables as mean and standard deviation. Differences in anthropometric and laboratory values between the two groups were assessed by analysis of covariance (ANCOVA) adjusted for sex, age, Tanner stage, time interval between visits (in months) and baseline value of the analyzed parameter. We fitted a logistic regression model to analyze the variables “decrease in BMI of 5% or greater” and “decrease in BMI greater than 10%”. We assessed changes within groups in the prevalence of prediabetes and hypertension between T1 and T3 using the McNemar test for paired samples and in body composition variables using the Student t test for paired samples. We set the level of statistical significance at 5% for all tests. The data were analyzed with the statistical package IBM SPSS, version 26.0.
ResultsTable 1 summarizes the baseline characteristics of the sample at the beginning of the study. The proportion of male patients was 51.6%, and 84% were in Tanner stage 4 or 5. The degree of obesity was greater in the group treated with liraglutide, with a mean z score 6 (SD, 1.7) versus 4 (SD, 1.6) in the control group (P< .001), as was the waist-to-hip ratio (0.63 [SD, 0.01] vs 0.56 [SD, 0.11]). We did not find any differences in the remaining blood chemistry, body composition and extracurricular physical activity variables.
Description of the sample at baseline.
| Variable | Liraglutide (N=31) | No liraglutide (N=31) | P |
|---|---|---|---|
| Male sex | 16 (51.6%) | 16 (51.6%) | 1a |
| Age (years) | 14.25 (1.29) | 14.24 (1.30) | .913 |
| Tanner stage | |||
| 1, 2 or 3 | 5 (16.7%) | 5 (16.1%) | 1a |
| 4 or 5 | 25 (83.3%) | 26 (83.9%) | |
| Weight (kg) | 103.67 (15.41) | 87.44 (17.50) | <.001b |
| Height (cm) | 167.54 (7.23) | 166.48 (8.7) | .601b |
| BMI (kg/m2) | 36.91 (4.72) | 31.3 (4.32) | <.001b |
| BMIz | 6.03 (1.72) | 4.04 (1.56) | <.001b |
| Waist circumference (cm) | 105.54 (10.71) | 94.37 (11.44) | <.001b |
| Waist-to-height ratio | 0.63 (0.06) | 0.56 (0.06) | <.001b |
| Basal fasting glucose (mg/dL) | 88.19 (9.57) | 87.9 (7.36) | .894b |
| HbA1c (%) | 5.45 (0.31) | 5.41 (0.32) | .893b |
| Basal insulin, (mg/dL) | 23.63 (11.71) | 21.91 (9.59) | .552b |
| HOMA-IR | 5.19 (2.67) | 4.78 (2.14) | .536b |
| Total cholesterol (mg/dL) | 153.87 (3.00) | 159.34 (36.41) | .527b |
| LDL (mg/dL) | 92.16 (23.02) | 95.85 (32.62) | .617b |
| HDL (mg/dL) | 43.48 (6.96) | 43.56 (10.29) | .975b |
| Triglycerides (mg/dL) | 104.87 (54.90) | 108.13 (54.00) | .816b |
| Uric acid (mg/dL) | 5.82 (1.28) | 7.46 (5.03) | .089b |
| Extracurricular physical activity | 20 (66.7%) | 31 (67.7%) | .929a |
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance.
Data expressed as mean (standard deviation) except for the variables sex, Tanner stage and extracurricular physical activity, expressed as n (%).
Treatment with liraglutide was superior to LSM alone when it came to the decrease in BMIz, with a mean difference of −0.98 (95% CI, −1.57 to −0.39; P= .001). This corresponded to a mean decrease in the BMI of −2.38 (SD, 0.68) (P= .001), and a mean weight reduction of −5.5 kgs (SD, 1.84) (P= .004) in the treatment group. At the time of completion of treatment with liraglutide, 48.4% had achieved a reduction in BMI of at least 5%, while 29% had achieved a reduction of at least 10%, compared to 3% and 1%, respectively, in the group of patients that did not receive liraglutide, both of which were statistically significant differences (Fig. 1). In addition, the waist circumference changed by a mean of −7.36cm (SD, 1.50cm) in the liraglutide group compared to −3.095cm in controls, with a mean difference of −4.26 (95% CI, −7.97 to −0.56; P= .025), and a mean difference in the waist-to-hip ratio of −0.03 (95% CI −0.052 to −0.007; P= .012) (Table 2).
Changes in anthropometric variables.
| Variable | Difference between T2 and T1 | Liraglutide vs No liraglutide | Difference between T3 and T1 | Liraglutide vs No liraglutide | Difference between T3 and T2 | Liraglutide vs No liraglutide | |||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | |||||||
| Liraglutide (n=31) | No liraglutide (n=31) | Liraglutide (T3 n=23) | No liraglutide (T3 n=19) | Liraglutide (T3 n=23) | No liraglutide (T3 n=19) | ||||
| Weight | |||||||||
| Absolute change (kg) | −5.41 (1.57) | 0.108 (1.57) | −5.52 (−9.22; −1.82)* | −5.98 (2.77) | 0.53 (3.20) | −6.51 (−12.14; −0.88)* | −0.73 (1.70) | −0.79 (1.93) | 0.06 (−3.04; 3.13) |
| Relative change (%) | −4.92 (1.36) | 0.89 (1.40) | −5.81 (−9.13; −2.5)* | −5.63 (2.84) | 0.68 (3.28) | −6.30 (−12.07; −0.54)* | −0.69 (1.80) | −1.08 (2.05) | 0.39 (−2.89; 3.67) |
| BMI | |||||||||
| Absolute change (kg/m2) | −2.75 (0.56) | −0.37 (0.56) | −2.38 (−3.76; −1.00)* | −3.00 (1.00) | −0.72 (1.13) | −2.28 (−4.29; −0.27) | −0.58 (0.71) | −0.96 ± −2.58 | 0.38 (−0.9; 1.65) |
| Relative change (%) | −7.54 (1.53) | −0.49 (1.54) | −7.06 (−10.83; −3.29)* | −8.58 (3.04) | −1.69 (3.42) | −6.89 (−12.99; −0.79) | −1.76 (2.29) | −3.07 (2.57) | 1.31 (−2.8; 5.42) |
| BMIz | |||||||||
| Absolute change | −1.091 (0.24) | −0.10 (0.24) | −0.99 (−1.58; −0.4)* | −0.8 (0.43) | 0.12 (0.47) | −0.92 (−1.75; −0.09) | −0.02±0.3 | −0.2 (0.33) | 0.18 (−0.35; 0.70) |
| Relative change (%) | −19.67 (4.53) | 0.66 (4.44) | −20.334(−31.23; −9.41)* | −14.6 (9.63) | 5.35±10.53 | −19.95 (−38.59; −1.32) | 3.16 (9.41) | −4.57±10.37 | 7.723 (−8.92; 24.37) |
| Waist circumference (cm) | −7.36 (1.51) | −3.1 (1.55) | −4.27 (−7.97; −0.57)* | −8.48 (3.36) | −3.67 (3.40) | −4.80 (−10.31; 0.7) | −0.10 (2.39) | −1.10 (2.44) | 0.99 (−2.47; 4.47) |
| Waist-to-height ratio | −0.052 (0.009) | −0.022 (0.009) | −0.03 (−0.052; −0.007)* | 0.055 (0.02) (T3 n=20) | −0.025 (0.02) | −0.03 (−0.062; 0.003) | 0.002 (0.015) | −0.006 (0.015) | 0.008 (−0.014; 0.029)* |
Abbreviation: BMIz, body mass index z score; T1, start of study; T2, end of pharmacological treatment; T3, end of study.
We present the mean size of the changes and the mean difference in changes between groups accompanied by the standard error of the mean (SEM), estimated in the covariance analysis (ANCOVA) adjusted for sex, age, Tanner stage, months elapsed between visits and baseline value of the dependent variable. In the case of the waist circumference and waist-to-height ratio, the number of patients in the analysis was n=30 at T1 and n=20 at T2 and T3.
After a mean of 6.1 months (SD, 3.5) elapsed after completion of treatment with liraglutide, there were no significant differences between the groups in the BMIz, BMI, weight and waist-to-hip ratio values (Table 2).
Time (T3 vs T1)At the time of the last visit, weight loss was maintained in the liraglutide group (BMIz, −0.8 [SD, 0.42] vs 0.11 [SD, 0.46]) with a mean difference of 0.91 (95% CI, −1.7 to −0.08; P= .032) (Table 2). We did not observe changes in linear growth.
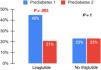
Cardiometabolic markersIn the liraglutide group, compared to the control group, there were significant decreases in insulin levels (mean difference, −8.87; 95% CI, −16.6 to −1.18; P= .025), the HOMA-IR (mean difference, −2.05; 95% CI, −3.8 to −0.29; P= .023) and triglyceride levels (mean difference, −49.87 [95% CI −81.54, −16.74]; P= .004), without significant differences in any of the remaining laboratory values under study (Table 3). There was a 46.2% reduction in the number of prediabetic patients in the liraglutide group, while the proportion remained the same in the control group (Fig. 2). The number of patients with hypertension decreased in both groups, and the decrease was only significant for systolic HTN (P= .031) in the liraglutide group (Fig. 3).
Estimated mean change in blood chemistry values between T1 and T3.
| Variable | Liraglutide (n=x) | No liraglutide (n=x) | Liraglutide vs No liraglutide |
|---|---|---|---|
| 95% CI | |||
| Basal fasting glucose | −2.70 (1.65) (n=28) | −2.44 (1.65) (n=27) | −2.66 (−3.86 to 3.33) |
| HbA1c (%) | −0.28 (0.08) (n=28) | −0.15 (0.07) (n=26) | −0.13 (−0.29 to 0.03) |
| Basal insulin, (mg/dL) | −9.60 (3.57) (n=27) | −0.73 (3.55) (n=23) | −8.87 (−16.56 to −1.18)* |
| HOMA-IR | −2.45 (0.81) (n=26) | −0.39 (0.80) (n=22) | −2.05 (−3.80 to −0.30)* |
| Total cholesterol (mg/dL) | −0.69 (5.82) (n=29) | 8.03 (5.78) (n=27) | −8.72 (−21.39 to 3.96) |
| LDL (mg/dL) | 0.98 (3.46) (n=29) | 2.40±3.5 (n=24) | −1.42 (−8.91 to 6.07) |
| HDL (mg/dL) | 4.00 (1.71) (n=29) | 3.17 (1.74) (n=25) | 0.84 (−2.85 to 4.53) |
| Triglycerides (mg/dL) | −44.53 (4.98) (n=29) | 4.62 (14.89) (n=28) | −49.15 (−81.55 to −16.74)* |
| Uric acid | −0.06 (0.36) (n=26) | 0.218 (0.32) (n=26) | −0.28 (−0.99 to 0.43) |
Abbreviation: HbA1c, glycated hemoglobin; HOMA-IR, Homeostatic Model Assessment for Insulin ResistanceT1, start of study; T2, end of pharmacological treatment; T3, end of study.
We present the mean size of the changes and the mean difference in changes between groups accompanied by the standard error of the mean (SEM), estimated in the covariance analysis (ANCOVA) adjusted for sex, age, Tanner stage, months elapsed between visits and baseline value of the dependent variable.
Changes in HTN.
The P value refers to changes within the group calculated with the McNemar test for paired samples. In the case of the change from the initial to the final frequency of systolic HTN, we found a significant difference between the liraglutide and no liraglutide groups (P= .031). The difference was not significant for diastolic HTN (P= .453). HTN, hypertension.
Due to technical problems in the BIA, we only show the data for a subset of 12 patients in each group. We observed a decrease in FM and FFM in the comparison of T3 vs T1 in both groups, although it was not statistically significant (Table 4).
Changes in bioelectrical impedance analysis variables by group (Liraglutide vs No liraglutide).
| Variable | Liraglutide (n=12) | No liraglutide (n=12) | ||||
|---|---|---|---|---|---|---|
| Baseline mean | Difference of baseline and final means | P* | Baseline mean | Difference of baseline and final means | P* | |
| Resistance (Rz) (Ohm) | 469.83 (70.07) | −2.6 (65.36) | .893 | 482.78 (100.26) | −1.4 (50.9) | .925 |
| Reactance (Xc) (Ohm) | 48.98 (9.95) | −3.57 (11.64) | .31 | 55.55 (8.25) | 0.01 (6.92) | .993 |
| Fat-free mass (FFM) (kg) | 63 (8.99) | −0.37 (6.51) | .849 | 59.2 (15.38) | −0.53 (4.59) | .695 |
| Fat mass (FM) (kg) | 32.62 (7.46) | −0.31 (4.99) | .83 | 26.39 (7.6) | −1.75 (3.86) | .145 |
| Body cell mass (BCM) (kg) | 33.88 (8.72) | −1.32 (6.69) | .507 | 34.07 (11.59) | −0.37 (6.39) | .843 |
| Phase angle (PA) (°) | 6.058 (1.4) | −0.31 (1.26) | .4 | 6.76 (1.6) | 0.05 (0.08) | .894 |
The most frequent adverse events were gastrointestinal, especially abdominal pain, which led five patients to discontinue treatment. Eleven patients reported difficulties with adhering to treatment consistently due to shortages in pharmacies and/or the financial cost to the family. The study did not involve blood glucose monitoring. None of the patients reported symptoms of hypoglycemia.
DiscussionIn our study in adolescent patients with obesity, treatment with liraglutide combined with LSM achieved a significantly greater reduction in the BMIz compared to the group not treated with liraglutide. This reduction was maintained up to 6 months after treatment completion, compared to a slight increase in the control group (Fig. 4). In the sole randomized controlled trial that has studied the effects of liraglutide in obese adolescents (125 assigned to liraglutide and 125 to placebo for a 56-week treatment period), conducted by Kelly et al in 2020,10 there was a significantly greater improvement in the BMIz in the liraglutide group compared to the placebo group (change of −0.23 [SD, 0.05] vs 0.00 [SD, 0.05]). In line with observations from trials in adults, weight regain was observed in the 26-week follow-up period after treatment completion. Tamborlane et al analyzed the effect of liraglutide combined with metformin on HbA1 levels in a sample of adolescents with excess weight and T2D, with 66 assigned to liraglutide and metformin and 66 to placebo and metformin for a duration of 26 weeks.11 The decrease in the BMIz was of −0.25 in the liraglutide group and −0.21 in the placebo group, a difference that was not significant. In a study conducted by Zhou et al in patients with prediabetes and obesity with a mean age of 11 years to assess the effect of liraglutide on glycemic control, there was a significant decrease in BMI at 3 months in the intervention group (n=21) compared to the placebo control group.12 Another two randomized, double-blind placebo-controlled trials whose purpose was to assess the safety, pharmacokinetics and side effects of liraglutide, one including 24 patients aged 7 to 11 years13 and the other 12 patients aged 12 to 17 years,14 found reductions in the BMIz of −0.28 (SD, 0.09) after 8 weeks of treatment and −0.02 (SD, 0.07) after 5 weeks of treatment, respectively.
Changes in anthropometric values during the study.
(A) Changes in the BMI z score in each group. (B) Changes in BMI in each group. (C) Changes in weight in each group. BMI, body mass index; CI, confidence interval; T1, beginning of study; T2, end of pharmacological treatment; T3, end of study.
In the pediatric age group, the amount of weight that needs to be lost or not further gained to improve the BMI and associated complications has not been clearly established. A systematic review by the United States Preventive Services Task Force15 on the impact of the treatment of obesity in children and adolescents found that a change in the BMIz of −0.20 was clinically significant and that decreases ranging from 0.20 to 0.25 are already thresholds for improving cardiometabolic risk factors, with greater improvement with decreases greater than 0.5. A Cochrane review found that lifestyle modification interventions (exercise and nutrition education)16 were associated with a change in the BMIz of −0.06. In our study, a change of −1.09 in the BMIz was associated with a significant decrease in insulin levels, HOMA-IR score, triglyceride levels and systolic HTN in the liraglutide group compared to the control group. Although there was a small, nonsignificant decrease in HbA1c in both groups, the percentage of patients with prediabetes decreased by 46% in the liraglutide group while it remained stable in the control group. In the 12-month follow-up of 88 adolescents with obesity, Ford et al17 also observed that decreases in the BMIz of 0.5 or greater were associated with a reduction in triglycerides (−30%), LDL (−15%) and high-sensitivity C-reactive protein (−45%), whereas smaller decreases (decrease in BMIz ≥ 0.25) improved insulin sensitivity, blood pressure and the total cholesterol-to-HDL ratio. The greater the decrease in BMIz, the greater the reduction in waist circumference and in the total body and truncal fat percentage estimated by means of BIA.
In seven randomized trials with a cumulative total of 547 participants,18–22 when the primary endpoint of the study was the decrease in BMI, there were no significant changes in cardiometabolic markers between groups, whereas when the primary endpoint was the change in blood glucose and/or HbA1c in patients with prediabetes or T2D, there was a significant reduction in these markers, although not in the lipid profile or blood pressure values. Recently, in a retrospective study in 24 adolescents with severe obesity treated with liraglutide for 3 months, Apperley et al found a significant reduction of −0.09 in the BMIz, in addition to decreases in triglyceride, cholesterol and HbA1c values and improvements in body composition and quality of life.23,24
In the analysis of body composition of our patients, we found a nonsignificant decrease in FM and FFM in both groups. The interpretation of these data is complicated, given the small sample size. The use of GLP-1RAs, such as liraglutide, achieve weight loss but also a rapid and significant loss of fat-free mass, and, while the evidence is still scarce, resistance training is recommended to minimize lean mass losses.25 In our study, we only assessed the practice of extracurricular physical activity in terms of intensity and frequency (hours/week), as self-reported by the patients, and while we found greater physical activity in the liraglutide group at T3 (data not shown), it was an indirect estimate, as we did not use precise measurement tools nor assessed the type of physical activity practiced by patients.
In 2023, the American Academy of Pediatrics published its first evidence-based clinical practice guideline for the evaluation and treatment of pediatric overweight and obesity, which recommends the use of medication in adolescents aged 12 or more years with a BMI at or above the P95.26 Several key trials of drugs for treatment of obesity in adolescents were published almost simultaneously. At the time of this writing, the FDA has approved liraglutide, semaglutide, phentermine/topiramate and setmelanotide (in addition to the already approved orlistat) for use in the pediatric population. The EMA has authorized liraglutide and semaglutide (2024) in children from age 12 years with a BMI at or greater than the P95, and setmelanotide from age 2 years (2025) for treatment of monogenic obesity. But initiating pharmacological treatment in real-world practice depends not only on the age and BMI of the patient, but also on the changes in BMI over time, the presence of comorbidities, the response to LSM and the preferences of the patient and/or family. Since most adolescents with severe obesity do not achieve a clinically significant decrease in BMI with LSM alone, it seems reasonable to offer medication as adjuvant therapy. Thus, an appropriate strategy in clinical practice could be, for example, trying LSM interventions for at least 4 to 6 weeks and assess the response prior to initiating pharmacological treatment. In our study, families chose to initiate liraglutide more chiefly in patients with greater degrees of obesity and progressive weight gain in spite of the recommended LSM measures. The severity of obesity is not a predictor of the effectiveness of liraglutide in either adult or pediatric patients,27 but only of the initial response in the early weeks of treatment. Treatment continuation depends on the response to the drug and whether the family can afford it. If there is no response after 3 months, treatment is discontinued. The most frequent adverse events associated with liraglutide are gastrointestinal (abdominal pain, nausea, vomiting, etc), which led to discontinuation of treatment in five of our patients. In the trial conducted by Kelly et al,9 gastrointestinal events were the reason for treatment discontinuation in 10 out of the 13 patients in whom it was discontinued.
Although the evidence supporting the safety and efficacy of anti-obesity drugs in the pediatric population is rapidly growing, there is still a considerable mismatch between the high prevalence of obesity and the infrequent utilization of these drugs in this age group. The clinical trials published to date analyzed their use for less than 2 years, and we do not know their potential impact on the long-term physiological and psychological development of the child. Another aspect that limits the use of these drugs is the notion that children with obesity can achieve a normal weight simply by refraining from consuming unhealthy foods and engaging in physical activity. This belief leads to the assumption that medication is unnecessary and even dangerous, a belief that is not only held by parents28 but is also shared by some health care professionals. Last of all, the lack of public funding also limits access to these drugs,29 further widening the social gap in obesity, with a higher prevalence in low-income families.
Among the limitations of our study, we ought to highlight the amount of missing data for anthropometric variables at T3 (21% in treatment group and 39% in control group). Although LSM measures were reviewed monthly by a dietitian, the proportion of patients lost to follow-up was greater in the control group due to poorer outcomes in these patients, who dropped the treatment. Discontinuation of liraglutide led to losses in the treatment group. One of the greatest challenges in the treatment of obesity is the high rate of attrition, which ranges between 40% to 70% in the published literature.2 The allocation to the groups depended on the ability of families to choose liraglutide, in addition to stocking issues in pharmacies, so we adjusted the analysis for the baseline values of the anthropometric measurements and other variables that could have an effect on the outcome variables. In addition, the Bodygram software required to capture and process BIA data was down for some time in addition to some other technical problems during the study period. When the distributor updated the software, the system had to switch from Bodygram PLUS to Bodygram HBO.
In conclusion, the use of liraglutide combined with LSM in a sample of adolescents with severe obesity achieved a greater mean reduction in the BMIz (−1.09 [SD, 0.24] vs −0.10 [SD, 0.25]) and the waist-to-height ratio compared to the control group managed with LSM alone. This weight loss was accompanied by a significant decrease in triglyceride levels, the HOMA-IR, insulin, systolic HTN and number of prediabetic patients compared to the control group. Prior to the addition of liraglutide, many patients continued to gain weight despite the LSM recommendations. The use of GLP-1RAs has had an important therapeutic impact in the management of obesity, as these drugs reduce appetite by delaying gastric emptying and promote satiety through their effects on the central nervous system. More studies are required to assess the long-term effects of these drugs on the physical and psychological development of the child, considering pediatric obesity a chronic disease that can recur, as opposed to the result of a lack of willpower in changing behavior.
Declaration of Generative AI and AI-assisted technologies in the writing processDuring the preparation of this work the authors used ChatGPT in order to generate the abstracts of the article in Spanish and English. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
FundingThis research project did not receive specific financial support from funding agencies in the public, private or not-for-profit sectors.
The authors have no conflicts of interest to declare.