Growth in patients with isolated growth hormone (GH) deficiency is heterogeneous despite treatment due to the low specificity of diagnostic tests, making it necessary to define efficacy variables.
AimsTo evaluate efficacy of hormone replacement therapy in children with isolated GH deficiency.
MethodsObservational-ambispective study of patients treated in our department in the last 14 years for isolated GH deficiency. This was defined as a GH level less than 7.4mg/dL in response to 2 stimulation tests in patients with height<2SD and a decreased growth rate.
ResultsThe study included a total of 97 patients, of whom 69% were boys. The large majority (89.58%) achieved final height. None of them had side effects. The median dose of GH used was 0.028mg/kg/day (0.03–0.025). There was a gain of 1.17 SD in final height. Around three-quarters (71.13%) of the patients were reassessed in adulthood, of whom 39.4% maintained the deficiency, and 79.31% achieved target range height. Target height, estimated height, and the total pubertal gain were positively correlated with final height, while the bone age/chronological age ratio and the initial insulin-like growth factor-1 showed a negative correlation.
ConclusionsA majority of patients reached target size, although only a few of them maintained the deficiency in adulthood. Target size, estimated adult height, and pubertal variables are directly related to adult height, while bone age/chronological age and insulin-like growth factor-1 were inversely related, and these can be used as efficacy variables. No adverse effects were observed in the sample with the doses used for the treatment.
El crecimiento en pacientes con déficit aislado de hormona del crecimiento (GH) es heterogéneo a pesar del tratamiento, debido a la baja especificidad de las pruebas diagnósticas, por lo que es necesario definir las variables de eficacia.
ObjetivosEvaluar la eficacia de la terapia de reemplazo hormonal en niños con déficit aislado de GH.
MétodosEstudio observacional-ambispectivo de pacientes tratados en nuestro servicio en los últimos 14 años por déficit aislado de GH, definido como GH inferior a 7,4mg/dl en 2 pruebas de estímulo, en pacientes con talla<−2DE y velocidad de crecimiento disminuida.
ResultadosSe estudiaron 97 pacientes. El 69% eran varones. Con el tratamiento hubo una ganancia de talla de 1,17DE. El 79,31% alcanzaron la talla diana. El 71,13% fueron reevaluados en la edad adulta, de los cuales el 39,4% mantuvo el déficit. La talla diana, el pronóstico de talla adulta y la ganancia puberal total se correlacionaron positivamente con la talla adulta, mientras que la relación edad ósea/edad cronológica y factor de crecimiento insulínico tipo 1 inicial mostraron una correlación negativa. Ninguno tuvo efectos secundarios.
ConclusionesLa mayoría de los pacientes alcanzaron la talla diana, aunque no todos mostraron permanencia del déficit en edad adulta. La talla diana, el pronóstico de talla adulta y las variables de pubertad están directamente relacionados con la talla adulta; mientras que la edad ósea/edad cronológica y factor de crecimiento insulínico tipo 1 están inversamente relacionadas, pudiendo utilizarse estas como variables de eficacia. No se han observado efectos adversos en la muestra con las dosis utilizadas.
Growth hormone deficiency (GHD) results in clinically significant growth retardation and metabolic disorders.1
Early diagnosis of GHD is important so that replacement therapy can be initiated as soon as possible with the aim of achieving an adult height approximating the target height (if GHD is not treated, the adult height can end up being 4.7 standard deviations [SD] below the mean) and prevent metabolic disorders in the long term.2
Since growth hormone (GH) secretion follows a pulsatile pattern, measuring its levels may not provide a reliable baseline, so instead pituitary stimulation tests are used to assess the secretion of GH.3,4 If there is no response, this finding must be confirmed by a second test using a different provocative stimulus due to the high proportion of false positives.4,5
Once GHD is diagnosed, treatment with recombinant human GH is initiated if the patient's height is 2 SD below the mean and the height velocity 1 SD below the mean.1
In Spain, treatment with GH is restricted to hospital settings and is overseen by the Advisory Committee for Growth Hormone Treatment of the competent autonomous community.6
Treatment is maintained until the height velocity decreases to less than 2cm/year and the bone age (BA) is greater than 16 years in male patients or 14 years in female patients. Nevertheless, GHD can continue to have an impact in adulthood due to its metabolic effects.7,8
Growth hormone replacement therapy carries significant costs for health care systems, so it would be beneficial to establish predictors of response to treatment in order to optimise its effectiveness and safety. This justifies the investment on long-term studies of GH replacement, which would provide clinicians the information needed to identify the patients that would most benefit from treatment without long-term risks.9
We aimed to conduct a study in our population to define GHD and the variables that influence adult height, for while similar studies have been conducted in countries such as Italy, France or the United States, none have been published in Spain on this subject.10,11
Thus, starting from the hypothesis that GH replacement increases adult height in Spanish patients with isolated GHD without a significant frequency of adverse effects, we set the objective of evaluating the effectiveness and safety of GH replacement therapy in patients with idiopathic isolated GHD, assessing the impact of pubertal development during treatment, the variables associated with effectiveness, and which patients continued to have GHD as adults.
Materials and methodsWe conducted a longitudinal retrospective and prospective descriptive study of children of both sexes aged 0–14 years given a diagnosis of GHD based on the results of 2 stimulation tests and that received GH replacement therapy until adulthood or the end of the period under in a tertiary hospital over a period of 14 years. In addition, after discontinuation of treatment upon reaching the adult height, all patients underwent a re-evaluation that included measurement of insulin-like growth factor 1 (IGF-1) and a stimulation test, for which we obtained their informed consent.
All anthropometric measurements were taken 3 times, with the patients barefoot and in their underwear, using a manual SECA scale accurate to 0.1kg and a wall-mounted Holtain stadiometer accurate to 0.1cm calibrated daily.
To make the diagnosis, 2 tests were performed on different days: the exercise provocation test, on account of the few adverse events associated with it, and a clonidine stimulation test; when it came to the re-evaluation, the stimulation test was performed with insulin. We did not use sex steroid priming. The thresholds established based on the current literature12 were 7.4ng/mL for the initial diagnosis and 5.6ng/mL for the re-evaluation. The levels of GH and IGF-1 were measured by qualified staff using a chemiluminescent immunoassay method (IMMULITE 2000).
The cohort was monitored from diagnosis until reaching the adult height, with collection of data for variables such as age at diagnosis, the SD of the weigh, height and body mass index at baseline and at 1, 2, 3 and 4 years of treatment, the bone age to chronological age ration (BA/CA) at baseline and at 1 year, predicted adult height (PAH) estimated according to the Bayley Pinneau method at baseline and at 1 year, IGF-1 at baseline and 1 year, target height, age at onset of puberty, height at onset of puberty (HOP), pubertal height gain (PHG), adult height and levels of GH and IGF-1 at the time of the re-evaluation.
Whenever possible, we adjusted the SD scores to the data of the cross-sectional growth studies conducted in Spain using the auxologic software AUXOTEC®,13 while levels IGF-1 were adjusted for age and sex using the software provided with the IMMULITE system.
Lastly, we performed a statistical analysis of the collected data with the software SPSS version 22. We compared 2 means using the Student t test if the variables followed a normal distribution or the Mann–Whitney U test otherwise and more than 2 means using ANOVA for normally distributed variables and the Kruskal Wallis test otherwise, and also calculated the Pearson correlation coefficient for comparison of quantitative data. We fitted a multiple regression model including the variables that had exhibited a significant association with final height in the previous analyses.
ResultsWe analysed the cases of 97 patients of both sexes with a diagnosis of GHD, treated with a mean dose of GH of 0.028mg/kg/day (0.025–0.03) for 5 years (from diagnosis to adulthood). The mean age at diagnosis was 10.5 years (8.26–11.7). Sixty nine percent of the patients were male, and 89.58% had achieved their adult height by the end of the study. We did not identify a neoplastic aetiology in any of the cases, and none of the patients experienced adverse effects from treatment.
Table 1 presents the main descriptive characteristics of the sample.
Descriptive statistics of the quantitative data collected during the followup through achievement of adult height in children with isolated growth hormone deficiency.
| Variables | n | Min | Max | Mean | SDS |
|---|---|---|---|---|---|
| Age at diagnosis (years) | 97 | 3.18 | 14.70 | 9.99 | 2.53 |
| Baseline height (SDS) | 97 | −5.91 | −1.78 | −2.80 | 0.62 |
| Baseline weight (SDS) | 97 | −3.20 | 0.83 | −1.25 | 0.65 |
| Baseline BMI (SDS) | 97 | −2.04 | 2.56 | −0.44 | 0.92 |
| Treatment dose (mg/kg/d) | 97 | 0.020 | 0.036 | 0.03 | 0.003 |
| Baseline IGF-1 (ng/mL) | 97 | 10 | 646 | 181.68 | 116.59 |
| Baseline IGF-1 (SDS) | 97 | −5.68 | 3.59 | −0.82 | 1.72 |
| Target height (SDS) | 94 | −3.09 | 1.33 | −1.37 | 0.77 |
| Bone age/chronological age | 97 | 0.29 | 0.96 | 0.71 | 0.17 |
| Baseline predicted adult height (SDS) | 97 | −4.28 | 2.99 | −1.55 | 1.44 |
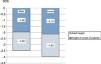
| Height at 1 year of treatment (SDS) | 97 | −3.31 | −1.19 | −2.30 | 0.49 |
| Weight at 1 year of treatment (SDS) | 97 | −2.07 | 0.63 | −1.13 | 0.59 |
| BMI at 1 year of treatment (SDS) | 97 | −1.63 | 2.16 | −0.49 | 0.83 |
| Bone age to chronological at 1 year of treatment | 97 | −0.12 | 1.05 | 0.77 | 0.19 |
| IGF-1 at 1 year of treatment (ng/mL) | 97 | 40 | 1347 | 378.54 | 228.17 |
| IGF-1 at 1 year of treatment (SDS) | 97 | −4.46 | 4.58 | 0.55 | 1.60 |
| Predicted adult height at 1 year of treatment (SDS) | 97 | −3.75 | 3.87 | −0.95 | 1.52 |
| Height at 2 years of treatment (SDS) | 97 | −3.66 | 0.32 | −1.98 | 0.60 |
| Height at 3 years of treatment (SDS) | 97 | −3.39 | 0.77 | −1.71 | 0.67 |
| Height at 4 years of treatment (SDS) | 96 | −3.09 | 1.28 | −1.59 | 0.78 |
| Adult height (SDS) | 82 | −3.76 | 0.93 | −1.64 | 0.86 |
| GH at re-evaluation (ng/mL) | 69 | 0.04 | 33.50 | 9.23 | 8.63 |
| IGF-1 at re-evaluation (ng/mL) | 82 | 23.0 | 1045.0 | 362.51 | 173.37 |
| IGF-1 at re-evaluation (SDS) | 82 | −7.99 | 3.74 | 0.02 | 2.01 |
BMI, body mass index; GH, growth hormone; IGF-1, insulin-like growth factor 1; SDS, standard deviation score.
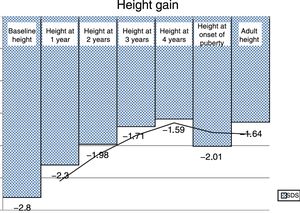
The mean height gain standard deviation score (SDS) in the first year of treatment was 0.49 SD score. The PAH adjusted for BA/CA increased by 0.6 SD. Fig. 1 shows the progressive improvement in height gain. The height SDS improved by 0.79 from baseline to puberty in both sexes, and from puberty to reaching the adult height by 0.43 in male patients and 0.29 in female patients. The total increase in height SDS until achievement of the final height was greater in male compared to female patients: 1.22 SD versus 1.08 SD (Fig. 2). The increase in the actual adult weight relative to the initial PAH was of 0.1 SD, and the increase relative to the target height was of 0.28 SD.
We found statistically significant differences (P<.05) based on sex in the variables shown in Table 2.
Statistically significant differences based on sex in the followup through adult height in children with isolated growth hormone deficiency.
| Boys | Girls | P | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SDS | n | Mean | SDS | ||
| Age at diagnosis (years) | 67 | 9.989 | 2.696 | 30 | 10.01 | 2.154 | NS |
| Baseline height (SDS) | 67 | −2.771 | .659 | 30 | −2.863 | 0.511 | NS |
| Baseline weight (SDS) | 67 | −1.259 | .681 | 30 | −1.246 | 0.598 | NS |
| Baseline BMI (SDS) | 67 | −0.418 | .941 | 30 | −0.478 | 0.873 | NS |
| Treatment dose (mg/kg/d) | 67 | ,0278 | .004 | 30 | 0.028 | 0.003 | NS |
| Baseline IGF-1 (ng/mL) | 67 | 164.5 | 123.4 | 30 | 219.4 | 90.99 | .002 |
| Baseline IGF-1 (SDS) | 67 | −1.209 | 1.849 | 30 | 0.026 | 0.951 | <.010 |
| Target height (SDS) | 67 | −1.253 | .773 | 30 | −1.618 | 0.700 | .027 |
| Bone age/chronological age | 67 | 0.683 | .171 | 30 | 0.777 | 0.147 | .006 |
| Baseline predicted adult height (SDS) | 67 | −1.236 | 1.368 | 30 | −2.237 | 1.366 | <.001 |
| Height at 1 year of treatment (SDS) | 67 | −2.327 | .523 | 30 | −2.256 | 0.421 | NS |
| Weight at 1 year of treatment (SDS) | 67 | −1.135 | .554 | 30 | −1.133 | 0.669 | NS |
| BMI at 1 year of treatment (SDS) | 67 | −0.464 | .830 | 30 | −0.553 | 0.844 | NS |
| Bone age/chronological age at 1 year of treatment | 67 | 0.750 | .201 | 30 | 0.813 | 0.160 | NS |
| IGF-1 at 1 year of treatment (ng/mL) | 67 | 335.3 | 199.2 | 30 | 473.6 | 260.8 | .016 |
| IGF-1 at 1 year of treatment (SDS) | 67 | 0.183 | 1.697 | 30 | 1.367 | 0.975 | .001 |
| Predicted adult height at 1 year of treatment (SDS) | 67 | −0.707 | 1.265 | 30 | −1.499 | 1.879 | .002 |
| Height at 2 years of treatment (SDS) | 67 | −1.959 | .643 | 30 | −2.037 | 0.503 | NS |
| Height at 3 years of treatment (SDS) | 67 | −1.689 | .744 | 30 | −1.759 | 0.458 | NS |
| Height at 4 years of treatment (SDS) | 67 | −1.548 | .835 | 29 | −1.690 | 0.655 | NS |
| Age at onset of puberty (years) | 67 | 12.54 | 1.197 | 30 | 11.79 | 1.084 | .006 |
| Height at onset of puberty (SDS) | 67 | −1.964 | .689 | 30 | −2.127 | 0.630 | NS |
| Adult height (SDS) | 59 | −1.533 | .937 | 25 | −1.834 | 0.596 | .034 |
| Pubertal height gain (cm) | 59 | 24.84 | 7.432 | 25 | 17.03 | 5.617 | <.010 |
| GH at re-evaluation (ng/mL) | 49 | 9.142 | 8.433 | 20 | 9.419 | 9.284 | NS |
| IGF-1 at re-evaluation (ng/mL) | 59 | 352.8 | 167.4 | 25 | 384.2 | 187.8 | NS |
| IGF-1 at re-evaluation (SDS) | 59 | −0.146 | 2.251 | 25 | 0.403 | 1.293 | NS |
BMI, body mass index; GH, growth hormone; IGF-1, insulin-like growth factor 1; NS, not significant; SDS, standard deviation score.
We found that adult height was associated with male sex, with male patients exhibiting greater heights throughout treatment and greater target heights, PAHs and PHG as well as earlier puberty, a lower BA/CA and lower levels of IGF-1 and GH in the re-evaluation (P<.05) (Table 3).
Association of variables analysed during the follow-up of children with isolated growth hormone deficiency with adult height.
| Variables | P |
|---|---|
| Age at diagnosis (years) | NS |
| Baseline height (SDS) | NS |
| Baseline weight (SDS) | NS |
| Baseline BMI (SDS) | NS |
| GH dose (mg/kg/d) | NS |
| Baseline IGF-1 (ng/mL) | NS |
| Baseline IGF-1 (SDS) | NS |
| Target height (SDS) | <.01 |
| Bone age/chronological age | .04 |
| Predicted adult height (SDS) | <.01 |
| Height at 1 year of treatment (SDS) | .003 |
| Weight at 1 year of treatment (SDS) | NS |
| BMI at 1 year of treatment (SDS) | NS |
| Bone age/chronological age at 1 year of treatment | .036 |
| IGF-1 at 1 year of treatment (ng/mL) | .006 |
| IGF-1 at 1 year of treatment (SDS) | .004 |
| Predicted adult height at 1 year of treatment (SDS) | <.01 |
| Height at 2 years of treatment (SDS) | <.01 |
| Height at 3 years of treatment (SDS) | <.01 |
| Height at 4 years of treatment (SDS) | <.01 |
| Height at onset of puberty | <.01 |
| Pubertal height gain | <.01 |
| Sex | <.01 |
BMI, body mass index; GH, growth hormone; IGF-1, insulin-like growth factor 1; NS, not significant; SDS, standard deviation score.
Lastly, we also observed a linear correlation between the body mass index and the dose of GH, and the dose of GH was higher at the end of treatment compared to the dose at treatment initiation (P<.05).
Re-evaluationWe re-evaluated 69 patients as adults (71.13%), and found that GHD persisted in 39.4%. Of all patients with persisting GHD, 69% were male.
We measured the levels of IGF-1 in the re-evaluation of adult height in 87 patients (89.58%), and found a mean level corresponding to a SDS score of 0.023, with deficient levels of IGF-1, defined as at least 2 SD below the mean, in 11%, all of whom also had deficient GH level in the re-evaluation (GH<5.4mg/dL) (P<.05). Although these differences were not statistically significant, we found that patients with permanent GHD had received the diagnosis earlier, had had lower baseline levels of IGF-1, lower height and weight at diagnosis, and exhibited a greater response to treatment.
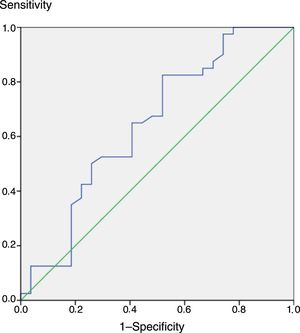
If in the re-evaluation we were to use a threshold for the IGF-1 levels of −3.22 SDS rather than −2 SDS, the area under the receiver operating characteristic (ROC) curve would have been of 0.8, which indicates that the test is good and shows a stronger correlation with GH (Fig. 3).
DiscussionAlthough GH has been used to treat short stature for over 40 years, there is no consensus on the criteria that should be applied to define a satisfactory response to treatment.14
One of the interesting aspects of our study was that nearly 90% of the recruited children achieved their adult height, so that we were able to re-evaluate a significant proportion of the patients to determine their evolution during treatment as well as the outcomes of treatment.
Other studies, like ours, reported diagnosis in prepubertal children with a predominance of the male sex, but with a mean age at diagnosis that is 2 years younger compared to our sample, where the mean age was 10.5 years, which could entail a poorer response to treatment.15
The published data are heterogeneous as regards the onset of puberty, with some studies reporting delays in the onset of puberty in patients with GHD and others no difference, as was the case of our cohort. On the other hand, the evidence currently available shows positive correlations between the age at diagnosis and puberty and between initial height, height gain at 1 year, age, sex, duration of treatment, BA/CA and adult height, of which our study only corroborated the association between age and diagnosis and age at onset of puberty, on one hand, and sex and height at baseline, height at 1 year and adult height, on the other.11,15–18 Maghnie et al. also reported an association between adult height and PHG,19 which has not been described by other authors, but which was consistent with our findings, in line with our objective of assessing the influence of puberty on adult height.
Furthermore, our analysis also revealed negative correlations between age at diagnosis and HOP, GH dose and HOP and the baseline level of IGF-1 and the PAH at 1 year, the HOP and the adult height, which had not been described to date. In addition, we found a statistically significant negative, rather than positive, correlation of BA with adult height, which seems logical given that greater delays in BA are associated with longer durations of growth, and that in the existing literature, duration of growth is either positively correlated with adult height or, as is the case of the Kabi International Growth Study (KIGS), not associated with it.20–22 Although there is no agreement between studies regarding the association of the age onset of puberty or the age at treatment initiation with adult height, we ought to mention that we did not find evidence of either.11,15–19,23,24
When it came to significant differences in height based on sex, we consider that while the literature and our own study have found an association between these variables,11,14 these differences may be due to differences between the sexes in pubertal growth and to the fact that target heights are lower in female patients, and, in our study in particular, that some of the female patients were in the prepubertal stage at the time of diagnosis (38% had the onset of puberty within 1 year). We also found negative correlations between age at onset of puberty and duration of puberty, on one hand, and PHG on the other, as described in previous studies.11,20,21
The KIGS found statistically significant associations of PHG with sex, target height, dose of GH, BA/CH, HOP and the age at onset and end of puberty,20,22 whereas our study only found a statistically significant positive correlation between PHG and male sex. The authors also noted that despite the intersubject variability in linear growth during treatment in patients of either sex in the prepubertal stage, there was no evidence of differences between the sexes in height gain and linear growth velocity in the first 3 years of treatment.21,25
There is also evidence of a negative association with the difference between the initial and the target height and between the peak value in the GH stimulation test, the age at diagnosis and adult height. The correlation between the peak GH value and adult height is also consistent with our findings of baseline GH values, age at diagnosis and a higher adult height, although in our study the differences were not statistically significant.11,16–19,23,24
The KIGS also reported an association of adult height with the frequency of GH injections, dose of GH, linear growth velocity in the previous year, current weight, birth weight and the severity of GHD. Neither our study or previous studies have found evidence of these associations, which denotes a need for further research on the subject.11,15–19,23
In addition, our study has also found associations between adult height and the initial PAH, the target height and the heights at different time points during treatment, including the baseline and the HOP, which had not yet been clearly established in the previous literature. Thus, adult height is associated with response to treatment throughout the duration of GH replacement therapy.
Lastly, while most authors assert that the target height is not associated with the adult height, probably due to samples including patients that could have idiopathic short stature, the Nordinet International Outcome Study (IOS) and the KIGS found a positive correlation between them, as was also the case in our study.11,15–19,23–25 Also in agreement with our study, Aimaretti et al. found an increase in the IGF-1 levels in association with treatment. These authors also reported a fair level of agreement between stimulation tests, the levels of IGF-1 and the diagnosis.26 In light of this, we ought to underscore our findings for IGF-1 and GH in the re-evaluation, which supports the agreement reported by this group and suggests a potential concomitant role of IGF-1 in the re-evaluation of GHD. In every case that the levels of IGF-1 were deficient in the re-evaluation, there had been no response in the GH stimulation test; however, not all patients with deficient GH values in the stimulation test had deficient levels of IGF-1 at re-evaluation, a fact that had not been clearly specified to present in the literature.27
Thus, in line with our set objectives, the published data and our experience, the target height, baseline PAH, HOP, age at onset of puberty, PHG, height gain at different points of treatment, BA/CA and level of IGF-1 could be predictors of the response to GH replacement therapy.
When it comes to efficacy, the studies in the literature have reported increases in the height SDS ranging from +0.4 to +1.5, in agreement with the value observed in our study, of +1.17 SDS.11,15,17,24 In addition, Ranke et al. reported a height gain of +1.2cm corresponding to +0.2 SDS at 1 year of treatment,28 slightly lower compared to our sample (+0.49 SDS). In our sample, this improvement in adult height fell within the normal range of the Spanish population, which was consistent with the findings of the KIGS.29
When it comes to the PHG, previous studies have reported a gain of +0.8 SDS compared to +0.43 SDS in our sample, which could be explained by the BA/CA and the short delay in the onset of puberty, resistance to treatment or lack of adherence, none of which we are able to confirm.15,30 Kirk also noted an improvement in height relative to the target height of between +0.2 and +0.8 SDS,16 and Blethen et al. reported an improvement relative to the PAH of +0.7 SDS,11 consistent with the improvement observed in our sample of +0.28 SDS relative to the target height, but with a significant discrepancy in regard to the improvement found in our sample in relation to the PAH, of only +0.1 SDS, which is likely related to the mild delay in BA in our sample.
Last of all, we ought to highlight that the proportion of patients with persistent GHD at the time of re-evaluation ranges from 44.4% to 71.9% in the literature, compared to 39.4% in our sample.12,26,31 We also found a younger age at diagnosis, lower baseline IGF-1 levels and baseline heights and a greater response to treatment in patients with persisting GHD. The predominance of the male sex is also worth noting, which may be associated with the higher frequency of abnormalities detected by magnetic resonance imaging at the time of diagnosis in these patients.
Although some authors have described adverse effects of treatment, Sotos and Tokar reported the absence of adverse events associated to treatment in their patients,32 which is consistent with the outcomes of GH replacement therapy observed in our patients and our hypothesis, although this could be due to differences in sample size. However, our results are also consistent with those of the study on the Safety and Appropriateness of Growth Hormone treatment in Europe (SAGhE) and the IOS, which had larger samples and longer durations of follow-up and found a low incidence of adverse events in these patients. Nevertheless, patients that have received this treatment in childhood should remain under follow-up to assess for potential long-term side effects that we may not yet be aware of.25,31,33–35 Lastly, the studies published to date on GH replacement therapy in children with isolated GH, with the exception of a randomised controlled trial published by Ranke et al.,36 are observational studies without controls in samples of 20–2852 children of both sexes with followup periods ranging from 1 to 9 years, that is, shorter than our study. Only 40% of these studies had larger samples compared to ours, and in some instances the samples were very small. We also ought to mention that most of the studies with samples larger than ours were sponsored by pharmaceutical companies, which was not the case of our study, as we did not receive any support and had no conflicts of interest.
That said, the factors that attest to the rigour of our study are its large sample size, the fact that it was conducted in a single centre, the careful methodology applied and the confirmation of statistical significance through the use of different tests.
On the other hand, the main limitations of our study are the missing data for variables whose data were collected retrospectively and the fact that while most patients had achieved their adult height by the end of the followup, it was not 100% of the sample. We also ought to consider the ever-present risk of measurement error and poor interrater reliability. Furthermore, GHD may have been overdiagnosed due to the limitations of the tests currently available for diagnosis, including the fact that we did not use sex steroid priming.
In short, in our sample the adult height after GH replacement therapy was similar to the target height, which supports our initial hypothesis regarding the effectiveness of this treatment. Of all patients, 39.4% exhibited persistent deficient levels of GH in the re-evaluation, of who 11.1% also exhibited deficient levels of IGF-1 at that time point. Furthermore, we found that patients with permanent GHD had received the diagnosis earlier, had had shorter stature and lower levels of IGF-1 at diagnosis and had exhibited a stronger response to treatment, which suggests that these patients may actually correspond to the true cases of GHD, although further research and better diagnostic tests are needed to confirm this hypothesis.
None of the patients experienced adverse events with the dosage used for treatment during the follow-up; and while we cannot establish with absolute certainty the safety of GH replacement therapy, our findings are supported by other studies in the literature.
Last of all, the association of different variables with the response to treatment suggest possible predictors for the effectiveness of treatment, such as the target height, the initial PAH, the age at onset of puberty, the PHG, the heights during treatment and the HOP, which are positively correlated with the adult height, and the BA/CA and baseline IGF-1 level, which are negatively correlated.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ariza Jiménez AB, Martínez-Aedo Ollero MJ, López-Siguero JP. Eficacia y seguridad del tratamiento sustitutivo en el déficit aislado de hormona del crecimiento. An Pediatr (Barc). 2019;90:285–292.