Primary nephrotic syndrome (NS) is a common glomerular disease in children. We assessed the genotypes and frequency of the rs5370 allelic variant of the EDN1 gene in children with primary NS.
Patients and methodsWe conducted a case-control study in Mansoura University Children's Hospital, Egypt between December 2015 and January 2018. We recruited 50 patients with steroid-sensitive NS (SSNS) and 50 patients with steroid-resistant NS (SRNS) in addition to 100 healthy controls. The patients underwent clinical evaluations and tests including measurement of serum albumin, cholesterol, creatinine and urea levels and a 24-h urinary protein test. We used polymerase chain reaction methods to assess the genotypes of rs5370 variants of the EDN1 gene (GG, GT and TT) and alleles (T and G) in the groups under study.
ResultsThe most frequent genotype of the EDN1 gene at the locus of interest in the control group was the GT genotype (88%; P=.001) while the GG genotype was more frequent in the NS group compared to the control group (P=.02). We did not find statistically significant differences between the NS and control groups in regard to the EDN1 rs5370 alleles (P=.69). The GG genotype was more frequent in the SSNS group compared to the SRNS and control groups (P=.03). When we compared allele frequencies between the control, SSNS and SRNS groups, we did not find significant differences (P=.89). The GT genotype was associated with normal blood pressure in children with NS (P=.007), while the GG genotype was associated with hypertension (P<.001). We did not find statistically significant differences in renal histopathology or serum cholesterol levels based on the genotype.
ConclusionsThe GG genotype at the rs5370 locus of the EDN1 gene may be associated with an increased risk of primary NS and a better response to steroid therapy.
El síndrome nefrótico (SN) primario es una glomerulopatía común en la edad pediátrica. Se evaluaron los genotipos y frecuencias alélicas del polimorfismo rs5370 del gen EDN1 en niños con SN primario.
Pacientes y métodosestudio de casos y controles realizado en el Hospital Infantil Universitario de El Mansura, Egipto, de diciembre de 2015 a enero de 2018. Se reclutaron 50 pacientes con SN corticosensible (SNCS) y 50 con SN corticorresistente (SNCR), así como 100 controles sanos. Se realizó evaluación clínica de los pacientes y pruebas de cuantificación de albúmina, colesterol, creatinina y urea séricas y de proteinuria en muestra de orina de 24 horas. Se emplearon técnicas de reacción en cadena de la polimerasa (PCR) para analizar los genotipos (GG, GT y TT) y los alelos (T y G) del polimorfismo rs5370 del gen EDN1 en los grupos bajo estudio.
ResultadosEl genotipo GT fue el genotipo más frecuente del polimorfismo rs5370 del gen EDN1 en el grupo de control (88%, p=0,001), mientras que el genotipo GG fue más frecuente en el grupo con SN en comparación con el de control (p=0,02). No se encontraron diferencias estadísticamente significativas entre los grupos de SN y de control en los alelos del polimorfismo rs5370 (p=0,69). El genotipo GG fue más prevalente en el grupo de SNSC en comparación con los grupos de SNRC y de control (p=0,03). Las diferencias en las frecuencias alélicas entre los grupos de SNRC, SNSC y de control no fueron significativas (p=0,89). El genotipo GT se asoció a una presión arterial normal en niños con SN (p=0,007) mientras que el genotipo GG se asoció a hipertensión (p<0,001). No se detectaron diferencias significativas en la histopatología renal o los niveles séricos de colesterol en base al genotipo.
ConclusionesEl genotipo GG del polimorfismo rs5370 del gen EDN1 podría asociarse a un riesgo mayor de desarrollar SN y una respuesta más favorable al tratamiento con corticoides.
Primary nephrotic syndrome (NS) is a common paediatric glomerular disease manifesting with generalised oedema, heavy proteinuria, hypoalbuminaemia and hyperlipidaemia, and its incidence is estimated at 2–7 cases per 100000 children.1
Endothelin-1 (ET-1) is a 21-amino acid growth-promoting peptide with a potent vasoconstrictor effect2 encoded by the EDN1 gene (6p24.1).3,4 There is strong evidence that ET-1 plays an important role in the pathogenesis of proteinuria and glomerulosclerosis through activation of the endothelin receptor type A, which causes oxidative stress and injury in the adjacent endothelial cells of glomeruli, leading to reciprocal paracrine injury to podocytes.5
Several studies have analysed the association between EDN1 rs5370 sequence variants and hypertension, systolic blood pressure and high-density lipoprotein cholesterol levels, but with conflicting results.6–11 When it comes to renal diseases, EDN1 variants have been associated with the progression of autosomal dominant polycystic kidney disease12 and IgA nephropathy.13 Few studies have been conducted to determine the impact of EDN1 sequence variants in children with NS.14 In our study, our main objective was to establish the genotypes and allele frequencies of EDN1 rs5370 sequence variants in children with primary NS. We also assessed the association between this single-nucleotide variation and the response to steroid therapy, blood pressure and serum cholesterol levels in these children.
Patients and methodsStudy design and participantsWe conducted a case-control study in Mansoura University Children's Hospital, Egypt between December 2015 and January 2018. We recruited 100 children with primary NS (50 with steroid-sensitive NS [SSNS] and 50 with steroid-resistant NS [SRNS]) and 100 healthy controls. We recruited controls in the same hospital among the patients presenting with minor complaints, such as pharyngitis and mild gastroenteritis, with normal blood pressure, urinalysis and kidney function test results and no history of renal disease. All participants had the same ethnic background. The study protocol was approved by the Institutional Research Board of Faculty of Medicine of Mansoura University, Egypt (Code Number: MS/15.09.47) and adhered to the Declaration of Helsinki of 1964 and subsequent amendments. We obtained written informed consent from the guardians of the cases and the controls.
Clinical evaluation and laboratory testsAll participants underwent a physical examination and anamnesis and collection of blood and urine samples for measurement of serum albumin, cholesterol and creatinine, a blood urea nitrogen test and a 24-h urine protein test. All cases had generalised oedema, a serum albumin level less than 2.5g/dL, proteinuria of 40mg/m²/hour or greater, and hyperlipidaemia.15 All study participants had an average body mass index (BMI) calculation between the 10th and 75th percentiles, as there may be an interaction between EDN1 rs5370 sequence variants and BMI.16 We calculated blood pressure values as the mean of 3 measurements, and classified blood pressure as normal if below the 90th percentile, prehypertension if between the 90–95th percentile and hypertension if above the 95th percentile for sex, age and height.17 We obtained data on renal histology and pathology from the health records of the patients (65 cases).
We defined SSNS as complete remission by 4 weeks of prednisone therapy at a dose of 60mg/m2/day, and SRNS as absence of complete remission after 4 weeks of prednisone therapy at 60mg/m2/day. We considered patients to be in remission if the level of albumin in urine was undetectable or there were just traces in 3 consecutive first morning specimens.18 We excluded patients with congenital or secondary NS, polycystic kidney disease, obesity or diabetes mellitus.
Genotyping of EDN1 rs5370 sequence variantsWe collected 3mL of venous blood from each participant using disposable plastic syringes and aseptic technique. We extracted genomic DNA from 200μL specimens of whole blood using Generation DNA Purification capture column kits (Fermentas, USA). We used polymerase chain reaction amplification-refractory mutation system (PCR-ARMS) for detection of EDN1 rs5370 sequence variants with the following primers19:
Generic reverse primer: 5′-AGTCAGGAACCAGCAGAGGA-3′
G allele primer: 5′-ATCCGAAGCTGAAAGGCAAG-3′
T allele primer: 5′-ATCCGAAGCTGAAAGGCAAT-3′
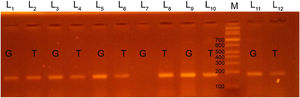
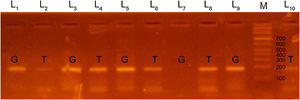
We used 2 Eppendorf tubes to prepare the PCR mixture. Each tube contained 3μL of DNA, 10μL of green PCR master mix (Fermentas) and 4μL of generic primer (10pmol/μL). Then we added 3μL of the specific primer (G or T, 10pmol/μL) in the tube. As for the PCR cycles, we started with a denaturation step at 95°C for 5min, followed by 35 cycles of 95°C for 60s, 58°C for 45s and 72°C for 45s, and a final extension step at 72°C for 5min. We then applied a holding temperature of 4°C. We performed gel electrophoresis of PCR products in 2.5% agarose gel. Lastly, we visualized the PCR products using ultraviolet light and ethidium bromide. We detected PCR products of EDN1 rs5370 sequence variants at the 184 base pair (bp) and photographed them with a digital camera.
We classified patients as having a GT genotype if two bands were detected at 184bp, one at the T primer lane and the other at the G primer lane. We classified patients as having a TT genotype when the patient had a single band at 184 bp in the T primer lane and no band in the G primer lane. Patients were considered to have a GG genotype if there was a single band in the G primer lane and no band in the T primer lane (Figs. 1, 2).
We analysed the data with the software SPSS version 23. We used the chi-square test to assess the agreement of genotypes and allele frequencies of EDN1 rs5370 variants with the Hardy–Weinberg equilibrium (P>.05 for each group). We compared categorical data (absolute frequencies and percentages) with the Fisher exact test and the chi-square test. We used the Kolmogorov–Smirnov test to assess the normality of the distribution in continuous data. We have expressed nonparametric variables as median and range (minimum-maximum) and compared them by means of the Kruskal–Wallis test. We defined statistical significance as a p-value of 0.05 or less.
ResultsPatients in all groups were matched for age, sex, residential setting (urban/rural), BMI percentile and blood pressure (Table 1). The distribution of genotypes at the EDN1 rs5370 locus was 18% GG, 70% GT and 12% TT in the NS group, and 7% GG, 88% GT and 5% TT in the control group. We found that the GT genotype was the most frequent genotype in the control group (88%; P=.001) while the GG genotype was more frequent in the NS group compared to the control group (P=.02) (Table 2).
Clinical characteristics of children with primary nephrotic syndrome and controls.
| Clinical characteristics | SSNS (n=50) | SRNS (n=50) | Control (n=100) | P |
|---|---|---|---|---|
| Age in years* | 8 (2.5−16) | 7 (3−17) | 6 (2−17) | .62 |
| Sex | ||||
| Male | 27 (54%) | 20 (40%) | 39 (39%) | .22 |
| Female | 23 (46%) | 30 (60%) | 61 (61%) | |
| Residential setting | ||||
| Rural | 29 (58%) | 34 (68%) | 66 (66%) | .52 |
| Urban | 21 (42%) | 16 (32%) | 34 (34%) | |
| Body mass index percentile | ||||
| 10th–25th | 4 (8%) | 6 (12%) | 16 (16%) | |
| 25th–50th | 19 (38%) | 18 (36%) | 42 (42%) | .5 |
| 50th–75th | 27 (54%) | 26 (52%) | 42 (42%) | |
| Blood pressure | ||||
| - Normal blood pressure | 40 (80%) | 36 (72%) | 100 (100%) | |
| - Prehypertension | 6 (12%) | 7 (14%) | 0 | .57 |
| - Hypertension | 4 (8%) | 7 (14%) | 0 |
Data expressed as n (%), except *median (minimum-maximum).
SRNS, steroid resistant nephrotic syndrome; SSNS, steroid sensitive nephrotic syndrome.
Distribution of genotypes and alleles of EDN1 rs5370 sequence variants in children with primary nephrotic syndrome and controls.
| NS group (n=100) n (%) | Control group (n=100) n (%) | P | |
|---|---|---|---|
| Genotypes (n) | |||
| GG | 18 (18%) | 7 (7%) | .02 |
| GT | 70 (70%) | 88 (88%) | .001 |
| TT | 12 (12%) | 5 (5%) | .07 |
| Allele frequency (2 n) | |||
| G | 106 (53%) | 102 (51%) | .69 |
| T | 94 (47%) | 98 (49%) | |
EDN1, endothelin-1 gene; NS, nephrotic syndrome.
When it came to the frequency of the EDN1 rs5370G and T alleles, we found no statistically significant differences between patients with NS and controls (P=.69) (Table 2). The binary logistic regression analysis revealed that the GG genotype was associated with a 3.23-fold risk of NS compared to the GT genotype (odds ratio, 3.23; 95% confidence interval [CI], 1.28–8.2).
When it came to the response to steroid therapy, we found that the GG genotype was more frequent in the SSNS group compared to the SRNS and control groups (P=.03). We found no significant differences in the allele frequency distribution between the control, SSNS and SRNS groups (P=.89) (Table 3).
Distribution of genotypes and alleles at the EDN1 rs5370 locus in children with steroid-sensitive nephrotic syndrome (SSNS), steroid-resistant nephrotic syndrome (SRNS) and controls.
| SSNS group (n=50) | SRNS group (n=50) | Control group (n=100) | P | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Genotypes (n) | ||||
| GG | 11 (22%) | 7 (14%) | 7 (7%) | .03 |
| GT | 32 (64%) | 38 (76%) | 88 (88%) | .002 |
| TT | 7 (14%) | 5 (10%) | 5 (5%) | .16 |
| Allele frequency (2 n) | ||||
| G | 54 (54%) | 52 (52%) | 102 (51%) | .89 |
| T | 46 (46%) | 48 (48%) | 98 (49%) | |
EDN1, endothelin-1; SRNS, steroid resistant nephrotic syndrome; SSNS, steroid sensitive nephrotic syndrome.
The GT genotype was associated with normal blood pressure in NS children (P=.007) while the GG genotype was associated with hypertension (P<.001). We did not find significant differences in serum cholesterol levels between the NS patient subgroups based on the genotypes under study (P=.068) (Table 4).
Distribution of genotypes of EDN1 rs5370 polymorphism according to blood pressure values and serum cholesterol levels in children with primary nephrotic syndrome.
| GG (n=18) | GT (n=70) | TT (n=12) | P | |
|---|---|---|---|---|
| Blood pressure values | ||||
| - Normal | 9 (50%) | 59 (84.3%) | 8 (66.7%) | .007 |
| - Prehypertension | 2 (11.1%) | 8 (11.4%) | 3 (25%) | .4 |
| - Hypertension | 7 (38.9%) | 3 (4.3%) | 1 (8.3%) | <.001 |
| Serum cholesterol levels* (mg/dL) | 377.5 (234-616) | 431.5 (214-686) | 486.5 (230-686) | .068 |
Data expressed as n (%), except *median (minimum-maximum).
When it came to renal histology and pathology (data available in 65/100 cases), we found no significant differences based on the EDN1 rs5370 genotype (Table 5).
Distribution of EDN1 rs5370 genotypes based on renal histopathology in 65 children with nephrotic syndrome.
| Renal histopathology | GG (n=11) | GT (n=50) | TT (n=4) | P |
|---|---|---|---|---|
| MCNS (n=51) | 8 (15.7%) | 41 (80.4%) | 2 (3.9%) | .55 |
| FSGS (n=8) | 1 (12.5%) | 6 (75%) | 1 (12.5%) | .69 |
| MP (n=6) | 2 (33.3%) | 3 (50%) | 1 (16.7%) | .43 |
FSGS, focal segmental glomerulosclerosis; MCNS, minimal change nephrotic syndrome; MP, membranoproliferative.
Primary NS is a common renal disease in the paediatric population. Although several studies have focused on the EDN1 gene as a susceptibility locus for chronic renal diseases in children, its exact role in NS is still unclear.20 We conducted a preliminary study to assess the impact of EDN1 rs5370 sequence variants in children with primary NS. The GG genotype at the EDN1 rs5370 locus was more prevalent in children with NS compared to healthy controls and associated with a 3.23-fold risk for NS. This finding suggests that the GG genotype may play a significant role in the pathogenesis of primary NS. EDN1 rs5370 sequence variations may result in a lysine-to-asparagine substitution at codon 198 (K198N), which would induce conversion of preproendothelin, thereby influencing ET-1 synthesis20 and causing structural changes in podocytes.21–23 However, this hypothesis needs to be corroborated by an analysis of the correlation of EDN1 rs5370 sequence variants with ET-1 levels in future studies. Furthermore, EDN1 mRNA studies are required to prove the functional effects of EDN1 rs5370 sequence variants. Our literature review yielded contradictory results. In a study conducted in China, Yang et al. reported a trend towards a difference in EDN1 rs5370 genotypes between 36 children with primary NS and 94 controls (P=.057).14 However, a more recent study conducted in Iran in 138 children with NS and 150 controls failed to confirm an association between EDN-1 rs5370 sequence variants and NS.24 Interethnic differences and sample sizes can have a significant effect on study results, which evinces the need for larger multicentre studies to clarify the potential role of sequence variations at this locus in the pathogenesis of primary NS.
In our study, we found significant differences in the frequency of GG and GT genotypes of the EDN1 rs5370 locus between the SSNS, SRNS and control groups. This suggests a possible role of EDN1 rs5370 sequence variations in the response to steroid therapy of patients with NS. This was consistent with the findings of by Ezzat et al., who reported that ET-1 and its receptors are involved in the effects of glucocorticoid therapy on different human cells, including those in the renal glomeruli.25 It was also consistent with the findings of Ahmed et al., who found an association between a high ET-1 level and a poor steroid therapy response in children with primary NS.26
Our study also suggested a potential association between the GG genotype and hypertension, while the GT genotype was more prevalent in children with NS that had a normal blood pressure. It is well known that ET-1 is a potent vasoconstrictor that can affect blood pressure.27 A study by Funalot et al. found that EDN1 rs5370 genotypes were not associated with blood pressure values, but that the interaction between this sequence variant and genes encoding endothelin-converting enzyme-1 could modulate blood pressure in women.28 Wiltshire et al. studied EDN1 rs5370 sequence variants in 1109 individuals in Western Australia and did not find a significant association between these variants and hypertension (P=.27).10 However, we ought to mention that the pathogenesis of hypertension in NS is multifactorial29 and that additional large-scale extension studies analysing more variables are needed to accurately establish the association between the EDN1 rs5370 locus and hypertension in children with primary NS.
In our study, we did not find a statistically significant association between EDN1 rs5370 genotypes and serum cholesterol levels, which was consistent with the findings of Wiltshire et al. and Hashemi et al.,10,24 although in contradiction to the findings of Pare et al. and Yang et al.11,14 Our study also found no association between EDN1 rs5370 genotypes and renal histopathology. This potential association has not been studied in the previous literature and further investigation is necessary to confirm our results.
ConclusionThe GG genotype in the EDN1 rs5370 locus is associated with an increased risk of primary NS in children and a better response to steroid therapy. Larger multicentre studies are needed to validate the findings of this study.
Limitations of the studyThe study was conducted in a single centre and the sample size was small. We also did not analyse the association with serum ET-1 levels.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Rizk H, Hammad A, El-Said A, Wahba Y. Polimorfismo rs5370 del gen de la endotelina-1 en el síndrome nefrótico primario: estudio de casos y controles. An Pediatr (Barc). 2021;95:406–412.