The objective of this study was to assess the evolution of stress in families of children and adolescents who start psychopharmacological treatment after being diagnosed with attention deficit hyperactivity disorder (ADHD), and the ability to detect this change using the FSI (Family Strain Index) questionnaire.
MethodologyForty-eight (48) specialists in child–adolescent psychiatry or neuropediatrics included 429 families of children diagnosed with ADHD, represented by the father, mother or guardian of the child. In the baseline visit, and at two and four months, the intensity of the symptoms of ADHD was evaluated using the Abbreviated Conners Scale, and family stress was evaluated using the FSI questionnaire.
ResultsThe following was observed: (a) an improvement in the overall FSI score and in all its dimensions (P<.001); (b) an improvement in the intensity of the symptoms of hyperactivity (Conners, P<.0001); (c) good agreement between these two scales at two months (R-intraclass 0.825, P<.0001) and at four months of followup (R-intraclass 0.784, P<.0001). Ninety seven point nine percent (97.9%) of the children or adolescents (420) received treatment with modified-release methylphenidate.
ConclusionsThere was a significant relationship between the positive evolution of symptoms in children with ADHD and the reduction of family stress, as evaluated by the FSI questionnaire, after starting psychopharmacological treatment. This study showed a great sensitivity to change in the clinical situation of patients with ADHD, evaluated through the stress it produces on its families. It is recommended to use this questionnaire as an indirect measurement of the repercussions of the disorder on the environment of the child with ADHD in terms of family stress.
El objetivo del estudio fue analizar la evolución del estrés en las familias de niños o adolescentes que comienzan tratamiento psicofarmacológico, tras ser diagnosticados de un trastorno por déficit de atención con hiperactividad (TDAH), y la capacidad de detección de este cambio mediante el cuestionario Family Strain Index (FSI).
MetodologíaCuarenta y ocho especialistas en psiquiatría infanto-juvenil o neuropediatría incluyeron 429 familias de niños diagnosticados de TDAH, representadas por el padre, la madre o el tutor del niño. En la visita basal, a los 2 y 4 meses, se evaluó la intensidad de los síntomas del TDAH mediante la escala de Conners abreviada, y el estrés familiar mediante el cuestionario FSI.
ResultadosSe observó: a) mejoría en la puntuación global del FSI y en todas sus dimensiones (p<0,001); b) mejoría en la intensidad de los síntomas de hiperactividad (Conners, p<0,0001); c) una buena concordancia entre las 2 escalas, a los 2 meses (R-intraclase 0,825, p<0,0001) y a los 4 meses de seguimiento (R-intraclase 0,784, p<0,0001). El 97,9% de los niños (420) recibieron tratamiento con metilfenidato de liberación modificada.
ConclusionesSe observó una correlación significativa entre la evolución positiva de los síntomas de los niños con TDAH y la reducción del estrés familiar evaluado mediante el cuestionario FSI, tras la instauración del tratamiento psicofarmacológico. Este estudio demostró una gran sensibilidad al cambio de la situación clínica de los pacientes con TDAH evaluado a través del estrés producido sobre sus familias. Se recomienda el uso de este cuestionario como medida indirecta de la repercusión del trastorno sobre el entorno del niño con TDAH en términos de estrés familiar.
Attention deficit hyperactivity disorder (ADHD) is the most frequent neurobehavioural disorder in children, and is characterised by a persistent pattern of inattention and/or hyperactivity and impulsivity that often gives rise to serious impairments in academic performance and social adaptive and behavioural functioning.1–3
Males are between three and six times more likely to suffer from ADHD than females, and the disorder affects 3–7% of school-aged children.4–9
Attention deficit hyperactivity disorder is frequently associated with other behavioural disorders and delays in language development and learning.10,11 The families of children with ADHD are also significantly affected emotionally and in their daily activities, family dynamics change, and caregivers experience disturbances2,4,12–15 that may even affect their daily activity and productivity at work.16,17
Several studies have demonstrated the impact of ADHD on the family in terms of routines, mental health, economic burden, and personal freedom and leisure.18–22 However, few studies have assessed the evolution of these parameters when treatment is initiated.
For this study, we chose the Family Strain Index (FSI) to assess the evolution of family stress. The ease of completion of this tool offers an advantage over other questionnaires. The FSI has demonstrated an excellent internal consistency (α=0.87) and we believed it could be used to evaluate changes in the family associated with the clinical course of patients with ADHD, which was the aim of our study.23
MethodsStudy design and ethical principlesWe conducted an observational, prospective, noncomparative multicentre study with a follow-up period of four months of duration between April and September of 2010. The study was approved by the ethics committee of the Hospital Clínic i Provincial de Barcelona (2009/5347). All parents/guardians agreed to participate in the study by signing a written informed consent, which was also obtained from children older than 12 years.
Assessment of the study objectivesThe intensity of the ADHD symptoms of the child or adolescent was assessed by means of the Abbreviated Conners Scale (ACS), which was completed by the parent or guardian and referred to the past four weeks. The ACS consists of 10 items with four possible answers (not at all, 0; just a little, 1; pretty much, 2; very much, 3). The sum of the scores of all 10 items yields the total score on the scale (0–30 points).
Family stress or strain was measured by means of the FSI in reference to the past four weeks, which was completed by the parent/guardian. The FSI consists of six items that assess two dimensions, the “emotional” dimension (items 1 and 3: 0–8 points) and the limitations to the family or “restrictiveness” dimension (items 2, 4, 5 and 6: 0–16 points). Each item is scored on a five-point scale (never, 0; almost never, 1; sometimes, 2; almost always, 3; always, 4) with the total score ranging between 0 and 24 points. Higher scores indicate greater impairment in family functioning. The emotional items assess the level of affective or emotional stress, and the restrictiveness dimension the limitations in the family's social activities.
Inclusion criteriaThe child psychiatrists and neuropaediatricians that participated as researchers selected the families, which were included by consecutive random sampling. The children could be of any race and sex, were between 6 and 17 years of age, and had a diagnosis of ADHD based on the criteria defined by the DSM-IV-TR.7 Inclusion in the study required not having received any previous treatment for ADHD. The inclusion of cases with depression, anxiety, tics, oppositional-defiant disorder (ODD), conduct disorder, learning disorder or disruptive behaviour disorders was allowed. Patients were given the standard psychopharmacological treatment.
We excluded families with institutionalised children, with more than one child with an ADHD diagnosis, or with at least one child with intellectual disability (IQ<70), psychosis, schizophrenia, bipolar disorder, autism or pervasive developmental disorder.
Statistical analysisWe performed a descriptive analysis of frequencies and percentages for qualitative variables, and mean±standard deviation, minimum, maximum and 95% confidence interval (95% CI) for quantitative variables. We performed comparisons using the χ2 for comparing proportions or Wilcoxon's test. We compared the mean scores in the ACS and the FSI questionnaire using Student's t-test or one-factor ANOVA with the Bonferroni or Games–Howell multiple-comparison correction when the factor had more than two categories. To analyse the concordance between the mean variations in the intensity of ADHD symptoms (ACS) and in the quality of life of families (FSI), we analysed the intraclass correlation coefficient (ICC) of individual measurements and of their means. We used multiple linear regression analysis to control for the effect of sociodemographic, anthropometric and clinical variables on the variability of symptom intensity and family stress. We set the level of statistical significance at .05. The statistical analysis was performed with the SPSS 14.0 software.
ResultsForty-eight researchers participated in the study and enrolled 429 families of children with an ADHD diagnosis. Nineteen families dropped out of the study: six dropped out voluntarily (1.4%), eight were lost to followup (1.9%) and five dropped out for other reasons (1.2%), amounting to a 4.4% attrition rate.
Sociodemographic dataTable 1 summarises the characteristics of the family members that completed the study. The mean age of participants was 41.5 years (95% CI, 40.7–42.2) and the median was 40.8 years (21–70 years).
Sociodemographic data of the family member that completes the survey and of the families.
| N | % | |
|---|---|---|
| Family member completing survey | ||
| Mother | 303 | 71 |
| Father | 77 | 18 |
| Legal guardian | 35 | 8.2 |
| Other family members | 12 | 2.8 |
| Not documented | 2 | 0.5 |
| Educational attainment | ||
| Primary | 158 | 37.4 |
| Secondary | 160 | 37.9 |
| Postsecondary | 93 | 22 |
| No studies | 11 | 2.6 |
| Not documented | 7 | 1.6 |
| Type of household | ||
| Two-parent | 310 | 72.9 |
| Single-parent | 64 | 15.1 |
| Blended family | 42 | 9.9 |
| Child in tutelage or living with other family members | 9 | 2.1 |
| Not documented | 4 | 0.9 |
| Employment status | ||
| Both parents employed | 226 | 53.6 |
| Only father employed | 128 | 30.3 |
| Only mother employed | 45 | 10.7 |
| Neither parent employed | 23 | 5.5 |
| Not documented | 7 | 1.6 |
Table 2 summarises the sociodemographic and clinical data of the children with ADHD. Their mean age was 10.4 years (95% CI, 10.1–10.6), and the median age was 10.1 (6–17 years). We did not observe any differences in age between males and females. Two hundred and thirty-six children (57.1%) were 6–10 years old, and 177 (42.9%) were adolescents aged 11–17 years.
Sociodemographic and medical data of the child or adolescent.
| N | % | |
|---|---|---|
| Sex | ||
| Male | 317 | 76.2 |
| Female | 99 | 23.8 |
| Not documented | 13 | 3.03 |
| Age | ||
| Children | 236 | 57.1 |
| Adolescents | 177 | 42.9 |
| Not documented | 16 | 3.7 |
| Number of siblings | ||
| Only child | 74 | 19.2 |
| One sibling | 199 | 51.7 |
| Two siblings | 79 | 20.5 |
| Three or more siblings | 33 | 8.6 |
| Not documented | 44 | 10.3 |
| Medical history | ||
| No history of interest | 387 | 90.2 |
| Other | 13 | 3.03 |
| Asthma | 11 | 2.6 |
| Atopic dermatitis | 4 | 0.9 |
| Allergy | 2 | 0.5 |
| Migraines | 2 | 0.5 |
| Diabetes mellitus | 2 | 0.5 |
| Neurofibromatosis | 2 | 0.5 |
| Dyslipidaemia | 1 | 0.2 |
| Coeliac disease | 1 | 0.2 |
| Familial Mediterranean fever | 1 | 0.2 |
| High blood pressure | 1 | 0.2 |
| Ventricular septal defect | 1 | 0.2 |
| Platelet abnormalities | 1 | 0.2 |
N, number of patients; %, percentage of patients.
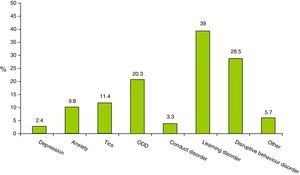
Psychiatric comorbidities were present in 28.7% (123) of the children, with 80.5% (99) having only one comorbidity, 18.7% (23) two comorbidities, and 0.8% (1) three comorbidities. Fig. 1 shows the proportions for each psychiatric comorbidity observed.
TreatmentAll children received psychoeducational therapy, and 97.9% (420) were treated with modified-release methylphenidate. Another three patients (0.7%) received methylphenidate combined with risperidone, and yet one other patient (0.2) methylphenidate in combination with tiapride. Five patients (1.2%) received atomoxetine as initial treatment for ADHD. The total daily doses ranged between 10 and 60mg of methylphenidate or 40 and 60mg of atomoxetine.
Family eventsForty-one families experienced family events that may have impacted their functioning and quality of life: parental separation (5), death in the family (6), parental disease or accident (8), parent becoming unemployed (7), aggression of family members by affected children (3) and other events (12).
Family Strain Index questionnaireTable 3 summarises the scores of the FSI questionnaire during the followup of the families.
Evolution of the scores in the Family Strain Index questionnaire and the Abbreviated Conners Scale during the followup.
| Dimensions of the FSI scale | Visits | N | Mean | Standard error | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Total scorea | Baseline | 394 | 13 | 0.279 | 12.5 | 13.6 |
| 2 months | 394 | 8.4 | 0.258 | 7.9 | 8.9 | |
| 4 months | 394 | 6.5 | 0.231 | 6 | 6.9 | |
| Emotional dimensiona | Basal | 394 | 4.9 | 0.907 | 4.8 | 5.1 |
| 2 months | 394 | 3.3 | 0.088 | 3.1 | 3.4 | |
| 4 months | 394 | 2.6 | 0.082 | 2.4 | 2.7 | |
| Restrictiveness dimensiona | Baseline | 394 | 8.1 | 0.201 | 7.7 | 8.5 |
| 2 months | 394 | 5.2 | 0.181 | 4.8 | 5.5 | |
| 4 months | 394 | 3.9 | 0.158 | 3.6 | 4.3 | |
| Abbreviated Conners Scale | Baselinea | 401 | 18.873 | 0.256 | 18.370 | 19.376 |
| 2 monthsa | 401 | 12.027 | 0.268 | 11.501 | 12.554 | |
| 4 monthsa | 401 | 9.399 | 0.256 | 8.896 | 9.902 | |
We observed statistically significant decreases in the total score and in every dimension of the FSI questionnaire between the three evaluation times (P<.0001).
In 353 families (87.6%), the FSI score improved between the baseline and the 2-month visits, and in 29 families (7.2%) it did not change. We observed that the total score of the FSI became worse in 21 families (5.2%).
Between the first and the four-month visits, the FSI score improved in 376 families (92.2%), it did not change in 18 families (4.4%), and it became worse in 14 families (3.4%).
We found no statistically significant differences in FSI scores based on the sex of the patient.
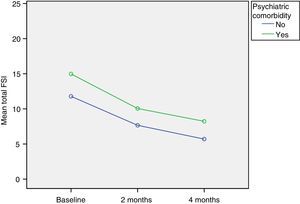
We found statistically significant differences in FSI scores between the families of children with psychiatric comorbidities and the families of children without psychiatric comorbidities (P<.0001), and the total scores of families of children with psychiatric comorbidities were significantly higher at every follow-up visit (Fig. 2). In the baseline visit, the average difference was of 3.2 points (95% CI, 1.8–4.6); at two months, the scores differed by 2.4 points (95% CI, 1.1–3.4) and at four months by 2.5 points (95% CI, 1.4–3.7). The differences persisted through the entire follow-up period. The differences continued to be significant when we performed the multivariate analysis to assess several possible risk factors in combination (P<.0001), in which the presence of psychiatric comorbidities was associated with a 2.7-point increase in the total score (95% CI, 1.5–3.9).
We found no interaction between sex and the presence or absence of psychiatric comorbidities. No other factor showed a significant association with the baseline score on the FSI questionnaire.
Abbreviated Conners ScaleTable 3 presents the results of the evolution of the scores.
We observed statistically significant improvements in the scores of the ACS in every consecutive follow-up visit (P<.0001). The score in the ACS improved in 377 patients (92.4%), and got worse between the baseline and the 2-month visit in 17 patients (4.2%) with the change amounting to one or two points in 11. The score did not change in 14 patients (3.4%). Between the baseline visit and the 4-month follow-up visit, the score improved in 390 patients (94.4%), got worse in 18 patients (4.4%) with changes amounting to 1 or 2 points in 14, and did not change in 5 patients (1.2%).
We found no differences in the ACS scores between boys and girls. The presence of psychiatric comorbidities was significantly correlated with the ACS scores through the entire follow-up period, with differences of 2.2 points in the baseline visit (95% CI, 1–3.6; P=.001), 1.6 points at 2 months (95% CI, 0.2–3; P=.027) and 1.9 points at 4 months (95% CI, 0.5–3.2; P=.006) between patients with comorbidities and patients without comorbidities, with the former having higher scores at every point in the followup. We did not find an interaction between these two factors and the ACS scores during followup. No other factor had a significant influence on the baseline score in the ACS.
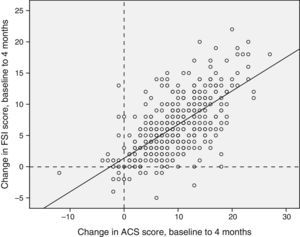
Correlation and concordance between the Conners Scale and the Family Strain Index questionnaire scoresWe found a statistically significant correlation between the two instruments (Fig. 3).
Concordance of the differences between the baseline and the third visits in the Conners scale and the FSI questionnaire. Concordance, 0.784 (95% CI, 0.737–0.822), P<.0001. Positive values indicate an improvement in family stress (FSI score) or ADHD symptoms measured by means of the Abbreviated Conners Scale.
The correlation coefficient for the baseline scores was 0.663 (Pearson's r; P<.0001). The correlation was still significant at the 2-month followup (Pearson's r, 0.731; P<.0001) and at 4 months (Pearson's r, 0.74; P<.001).
To study the sensitivity to change between the two assessment scales, we analysed the concordance of the increases in the scores of each questionnaire from the baseline visit to the 2-month followup and from the baseline visit to the 4-month followup, and we found an intraclass correlation coefficient of 0.825 (95% CI, 0.787–0.856; P<.0001) for the differences at 2 months, and of 0.784 (95% CI, 0.737–0.822; P<.0001) for the differences at 4 months.
DiscussionSeveral studies have demonstrated the negative impact of ADHD not only on the affected individual, but also on the other members of the family unit, as it is frequently associated with disrupted interpersonal relationships, a lower perceived family cohesion, greater conflict, and a higher incidence of depressive disorders and of separation and divorce in parents.23–26
The stressful and demanding behaviours of children with ADHD tend to evoke abnormal responses in other members of the family.13 The literature has reported a higher prevalence of depressive mood disorders,25–30 anxiety disorders30 and alcohol use29,31–34 in parents of children with ADHD than in parents of children without ADHD.
The stress experienced by parents of children with ADHD starts in early childhood. In a study conducted in children aged 3–5 years with ADHD compared to healthy controls, DuPaul et al. found that the parents of children with ADHD experienced greater stress, coped less adaptively and were more likely to display negative behaviour towards their children.35
Siblings of children with ADHD were also “victims” of the disorder. Kendall described that siblings felt victimised by the aggressive actions of the child with ADHD, as they were subject to physical violence, verbal abuse and manipulative and controlling behaviours. The siblings also reported that their parents expected them to care for and protect their siblings with ADHD because of the social and emotional immaturity associated with the disorder, and many of them described feeling anxious, worried and sad.36
Thus, the burden of caring for a child with ADHD often extends to every member of the household, giving rise to disrupted parent–child relationships that significantly reduce the quality of life of the members of the family.4,12,13,15,30 Parents end up feeling increasingly inadequate in childrearing, and all of it leads to decreased satisfaction in parenting.30 In fact, the literature has described poorer mother–child relationships and more punitive and negative parenting strategies towards children with ADHD.25,37
On the other hand, ADHD also seems to have a negative impact on both the work life and productivity of the parents. In a study by Noe and Hankin, 63% of caregivers reported some change in work status as a direct result of their child's ADHD: 15% had changed jobs, 46% had reduced the number of hours they worked, and 11% had stopped working altogether.16
In terms of costs, we ought to note that children with ADHD incur significantly greater per capita health care costs than children without ADHD. Guevara et al. found a difference of $1465 vs. $690, with 9.9 times more outpatient mental health visits (1.35/year vs. 0.14/year) and 1.6 more primary care visits (3.84/year vs. 2.36/year) by children with ADHD.38
Many studies have indicated that parent–child interactions improve significantly once the children are being treated with stimulant drugs. Even being informed of the ADHD diagnosis has a positive effect in itself on the stress and anxiety experienced by the family.39,40
In our study, the improvement in the ACS scores, which showed a reduction of 50% from 18.9 points in the baseline visit to 9.4 points in the final visit (P<.0001), indicates that children and adolescents suffering from ADHD benefitted from the initiation of psychopharmacological treatment, as it improved the clinical symptoms of ADHD. But the initiation of psychopharmacological treatment also brought forth a considerable reduction in the stress level of families, as evinced by the 50%, 53% and 48% decrease in the total, emotional dimension, and restrictiveness dimension FSI scores, respectively (P<.0001).
The mean family stress measured by means of the FSI questionnaire was higher in children in our study than in the children in other published studies. In the ADORE study, conducted on a sample of 1477 children aged 6–18 years, the FSI score was 10.27±5.41, compared to a stress score of 13 (95% CI, 12.5–13.6) in the children in our study. A possible explanation is that the ADORE study included children that were already undergoing treatment, so that family stress had already improved. If we calculate the mean stress at the three visits when treatment was already underway in our study, we obtain a mean FSI score of 9.3, a figure that is already comparable to the one found by the ADORE study.23
The presence of psychiatric comorbidities in ADHD patients was associated with a higher intensity of ADHD symptoms, as we observed that the scores in the ACS and FSI questionnaire were higher in these patients than in those that had no comorbidities. The difference persisted through the entire followup even as family stress and ADHD symptoms improved, with children with comorbidities continuing to show poorer outcomes than children without (Fig. 2).
The close association that exists between patient symptoms and family stress came to light when we analysed the correlation of the ACS and FSI scores, as we found a positive correlation both at the baseline visit (Pearson's r, 0.663; P<.0001) and at 2 and 4 months. The changes in both scales had a positive intraclass correlation during the followup, which shows that the parameters that they measure are clearly related (ICC, 0.825 [baseline to 2 months] and 0.784 [baseline to 4 months]; P<.0001).
One of the limitations of our study was that ADHD was diagnosed based solely on the DSM IV criteria. One of the greatest advantages of the study was the selection of naïve patients that had received no previous treatment, as it allowed us to make a much more reliable assessment of the impact of treatment on family stress levels. Future research could focus on continuing the followup of these families to assess whether stress continues to decrease overtime until it reaching normal levels.
We believe that the FSI questionnaire developed by Riley et al. is a very useful and easy to use instrument to assess the impact of ADHD on the family environment in terms of stress.23
We believe that the integrated management of ADHD must take into account not only the patient, but also the entire family. Reducing family and parent stress should be one of the goals of treatment, considering the central role of the parent and family in the child's upbringing.39
In short, early detection and appropriate treatment of this disorder can have a beneficial effect on the academic performance and psychosocial development of these patients and their impact on their family environment, an improvement that can be assessed by an instrument as simple as the FSI, which we evaluated in this study.
FundingAll phases of this study have been funded by JUSTE S.A.Q.F.
Conflicts of interestPilar García works for the medical department of the Juste S.A.Q.F. laboratories, which funded the study. Begoña Soler was responsible for the design and statistical analysis of the study as an outside consultant. The rest of the authors have no conflicts of interest to declare in relation to the objectives and results of this study.
We wish to thank the following participating researchers, listed here in alphabetical order, for their collaboration in the study:
Agüero Ramon-Llin, Cristina Celia; Albadadejo Gutierrez, Eloy; Almendral Doncel, Raquel; Arce Portillo, Maria Elena; Barroso Jornet, Jose Maria; Bermejo Gonzalez, Teresa; Blasco Herrera, Jose Maria; Casal Pena, Maria Cristina; Catala Ortuño, Elena; De Lucas Zaracena, Maria Teresa; Ferrer Carrio, Angels; Ferrer Gelabert, Roger; Fuentes Albero, Milagros; Gainza Tejedor, Ignacio; Gastaminza Perez, Xavier; Gomez Guerrero, Lorena; Gonzalez Collantes, Ruth; Gordo Seco, Rocio; Hernandez Gadino, Ana Maria; Hernando, Sara; Jarast Kaplan, Ricardo; Lafau Marchena, Oriol; Laguia Moreno, Carolina; Lara Herguedas, Julian; Luch Fernandez, Maria Dolores; Maside Miño, Elena; Mata Iturralde, Laura; Miravet Fuster, Elena; Montoliu Tamarit, Leonor; Ortega Garcia, Enrique; Parrilla Escobar, Maria; Perez, Ana; Perez Alvarez, Frederic; Pico Fuster, Gustavo; Pujals Altes, Elena; Rodrigo Gutierrez, Ana Cristina; Rodrigo Jimenez, Daniel; Rodrigo Matrene, Maria; Rodriguez Aisa, Beatriz; Romera Torrens, Maria; Rosal Roig, Jaume; Sanz De La Garza, Cesar Luis; Sasot Llevadot, Jordi; Vadillo Movellan, Enrique; Vazquez Lopez, Ester; Vega Fernandez, Flora; Yusta Izquierdo, Antonio.
Please cite this article as: Guerro-Prado D, Mardomingo-Sanz ML, Ortiz-Guerra JJ, García-García P, Soler-López B. Evolución del estrés familiar en niños con trastorno por déficit de atención con hiperactividad. An Pediatr (Barc). 2015;83:328–335.
Previous presentation: this study was presented as a poster under the name of Evolution of stress in families of children and adolescents with attention deficit hyperactivity disorder after psychopharmacological treatment 4-month follow-up study in Eunethydis; May 23–25, 2012; Barcelona, Spain.