The aim of this study is to review the current management and outcomes of foetal bradycardia in 9 Spanish centres.
MethodsRetrospective multicentre study: analysis of all foetuses with bradycardia diagnosed between January 2008 and September 2010. Underlying mechanisms of foetal bradyarrhythmias were studied with echocardiography.
ResultsA total of 37 cases were registered: 3 sinus bradycardia, 15 blocked atrial bigeminy, and 19 high grade atrioventricular blocks. Sinus bradycardia: 3 cases (100%) were associated with serious diseases. Blocked atrial bigeminy had an excellent outcome, except for one case with post-natal tachyarrhythmia. Of the atrioventricular blocks, 16% were related to congenital heart defects with isomerism, 63% related to the presence of maternal SSA/Ro antibodies, and 21% had unclear aetiology. Overall mortality was 20% (37%, if terminations of pregnancy are taken into account). Risk factors for mortality were congenital heart disease, hydrops and/or ventricular dysfunction. Management strategies differed among centres. Steroids were administrated in 73% of immune-mediated atrioventricular blocks, including the only immune-mediated IInd grade block. More than half (58%) of atrioventricular blocks had a pacemaker implanted in a follow-up of 18 months.
ConclusionsSustained foetal bradycardia requires a comprehensive study in all cases, including those with sinus bradycardia. Blocked atrial bigeminy has a good prognosis, but tachyarrhythmias may develop. Heart block has significant mortality and morbidity rates, and its management is still highly controversial.
Revisar el manejo actual y la evolución de la bradicardia fetal en 9centros españoles.
MétodoEstudio multicéntrico retrospectivo: análisis de todos los fetos con bradicardia diagnosticados en 9centros españoles entre enero de 2008 y septiembre de 2010. Los mecanismos electrofisiológicos responsables de la bradicardia fetal se estudiaron mediante ecocardiografía.
ResultadosSe registraron 37 casos: 3 fetos con bradicardia sinusal, 15 con extrasistolia auricular no conducida y 19 con bloqueo auriculoventricular (AV) de alto grado. Bradicardia sinusal: el 100% asoció patologías severas. Extrasistolia auricular no conducida: excelente pronóstico, pero un caso desarrolló posnatalmente taquicardia supraventricular. Entre los bloqueos AV de alto grado, el 16% asociaban cardiopatía congénita con isomerismo, el 63% anticuerpos antiRo/SSA maternos y el 21% fueron de etiología desconocida. La mortalidad global de los bloqueos AV fue del 20% (37% si consideramos la interrupción voluntaria del embarazo). Factores de riesgo fueron: asociar una cardiopatía congénita, hídrops y/o disfunción ventricular. El tratamiento fue variable según el centro, se administraron corticoides en el 73% de los bloqueos de gradoiii inmunomediados y en el único caso de bloqueo de gradoii inmunomediado. En un seguimiento medio de 18meses, se implantaron marcapasos en el 58% de los bloqueo AV de alto grado.
ConclusionesLa bradicardia fetal sostenida precisa siempre de un estudio exhaustivo, incluso en el caso de la bradicardia sinusal. La extrasistolia auricular no conducida tiene buen pronóstico pero puede asociar taquicardia. El bloqueo AV de alto grado fetal tiene todavía una morbimortalidad significativa y su tratamiento es controvertido.
Transient episodes of bradycardia are a common occurrence in the developing foetus and they are usually benign.1 However, sustained foetal bradycardia unrelated to labour is a rare but potentially serious entity. The condition may be due to sinus bradycardia (SB), blocked premature atrial contractions (BPACs), or high degree atrioventricular block (AVB).2 The latest pathology is very important due to its high mortality (17–43%)3–5 and morbidity,3 and to the international controversy surrounding its management.6 In most cases, in the absence of congenital heart disease (CHD), conduction defects are due to the transplacental passage of maternal antibodies that attack the conductive tissue with subsequent fibrosis,7 while in a smaller number of foetuses no cause is found for the block (non-immune or “idiopathic” AVB). In immune-mediated cases, the involved antibodies (anti-Ro/SSA) are necessary but not sufficient to cause disease,8 which affects only 1–2% of children of mothers with these antibodies (the risk is tenfold if the mother has a previous child with the condition).4 In addition to damaging the conductive tissue antibodies can cause diffuse cardiac damage in the form of endocardial fibroelastosis and dilated cardiomyopathy,9 which result in considerably worse outcomes. Different treatment approaches have been used in immune-mediated AVB, including fluorinated steroids, beta-agonists, and plasmapheresis, aiming to prevent myocardial inflammation, increase the foetal heart rate, and reverse heart failure,10–13 but their efficacy is controversial.6,14,15 The lack of consensus on the best strategy to manage these patients accounts for the heterogeneous, even divergent therapeutic approaches of different hospitals.15 This study describes the diagnosis and clinical approach to the management of foetal bradycardia in 9 Spanish hospitals, and aims for starting point for a future prospective multicentre study with a broader, nationwide scope.
ObjectiveIn 2008, the Foetal Cardiology group of the Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas (Spanish Society of Paediatric Cardiology and Congenital Cardiopathies [SECPCC]) decided to conduct a retrospective study to describe the diagnosis, current management, and outcome of foetal bradycardias.
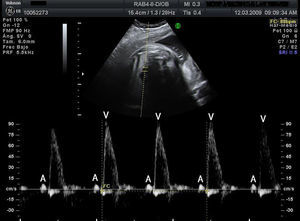
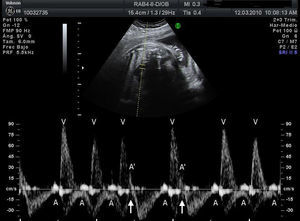
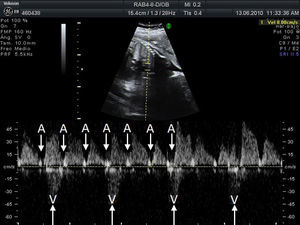
Materials and methodsWe conducted a retrospective, descriptive, cross-sectional, multicentre study to analyse the characteristics, treatment, and outcome of all foetuses diagnosed with bradycardia in 9 Spanish hospitals from January 2008 to September 2010 (20 months). To evaluate heart rhythm and the haemodynamic effects of bradycardia, we used M-mode echocardiography, pulsed wave Doppler, and tissue Doppler imaging of mitral inflow/aortic outflow, pulmonary vein/pulmonary artery, or superior vena cava/ascending aorta. Foetal bradycardia was defined as a sustained heart rate below 110beats per minute.16 It was defined as sinus bradycardia when the ratio of atrial to ventricular contractions was 1:1 (A:V=1:1) (Fig. 1), compared to bradycardia due to blocked premature atrial contractions (Fig. 2) with a bigeminal pattern, or to high-degree AVB (Fig. 3), in which the frequency of atrial contractions is greater than that of ventricular contractions (A:V>1). Blocked atrial bigeminy was defined as BPACs with an A:V ratio of 2:1 and varying time intervals between every 2 atrial contractions: one shorter than the interval characteristic of normal sinus beats, and one longer. The strategy, followed by participating centres in pregnant women who had anti-Ro and anti-La antibodies and whose foetuses were at high risk of developing AVB, was to perform an echocardiogram weekly from week 16 to week 28 to analyse the interval of time between atrial and ventricular contractions and detect any prolongation of this interval (suspected first-degree AVB).17 After week 28 through the end of the pregnancy, controls were performed every 4 weeks. Second-degree AVB was defined as having a constant interval between atrial contractions, conducted or not conducted to the ventricles intermittently or with a fixed A:V ratio (example 2:1 or greater). Third-degree AVB was defined as the complete dissociation of atrial and ventricular contractions, with constant intervals between atrial contractions. We recorded information on the diagnosis and therapeutic approach for each case: watchful waiting, intrauterine treatment, or delivery. The primary outcomes were the survival of the foetus and the efficacy of the treatment given (assessing reduction of the degree of AVB or haemodynamic improvement). We collected data on the antibody status of the mother, the association with structural cardiopathies, and the detection of signs of hydrops or ventricular dysfunction (VD). We documented the outcomes of the foetuses and the need for pacemakers in postnatal life. In the statistical analysis, we used Student's t test to compare means, and Fisher's exact test to analyse categorical variables. The level of statistical significance was set at P<.05.
A total of 37 patients were included: 15 foetuses with bradycardia due to BPACs, 3 with SB, and 19 with high-degree AVB.
Blocked premature atrial contractionsThe mean age at diagnosis was 27.5 weeks of gestational age (WGA) (standard deviation [SD]: 8.3). Outcomes were good, with spontaneous resolution of the arrhythmia, although 1 patient in whom a resting ECG at birth revealed Wolff–Parkinson–White syndrome had a postnatal episode of supraventricular tachycardia.
Sinus bradycardiaThe mean age at diagnosis was 25 WGA (range, 22–32; SD, 5.2). Parents chose to terminate pregnancy in 1 case in which the foetus had XO monosomy, omphalocoele, and hydrops. Another foetus received a diagnosis of mitochondrial disease after birth and developed severe neurologic abnormalities. The third foetus, whose mother was positive for anti-Ro/anti-La antibodies, alternated episodes of bradycardia and tachycardia, received a diagnosis of sick sinus syndrome at birth, and required a pacemaker.
High degree atrioventricular blockThe mean age at diagnosis was 23.5 WGA (range, 14–31; SD, 4.3), and in cases associated with CHD was 18 WGA, and 23.9 WGA in cases with no CHD. Table 1 summarises data on the diagnosis, management, and outcome of each foetus. Table 2 shows the analysed data, both overall and for each subset. Of the 19 foetuses, 2 (10%) had second-degree AVB, and 17 (90%) had third-degree AVB. Congenital heart disease was detected in 3 foetuses (16%), and was severe and associated to isomerism in all. Maternal antibodies were found in 12 (63%). The aetiology was unknown in the remaining 4 (21%). The overall mortality was 20% (or 37% if we include voluntary terminations of pregnancy [VTP]). We also analysed mortality in each subset. All 3 patients with AVB associated to CHD died (1 spontaneously and 2 by VTP). There was 1 death in the group of AVB of unknown aetiology, a foetus with hydrops and severe VD born at week 27 of gestation. Three (25%) of the 12 foetuses positive for antibodies died, 2 by VTP, and 1 spontaneously.
Nineteen foetuses with high-degree atrioventricular block.
| Foetus, Deg. AVB | WGA at Dx. | VR | Hydrops, VD | Immunol. CHD | IU tx | WGA at delivery | Outcome | DFU |
| 4: 2nd | 25 | 75 | No | Ab− | No | 37, C | PM− | 12min |
| 5: 2nd | 22 | 75 | No | Ab+ | Ster | 39, C | PM+ | 39min |
| 6: 3rd | 30 | 57 | No | Ab− | No | 40, C | PM− | 12min |
| 7: 3rd | 22 | 70 | No | Ab− | No | 38, C | PM+ | 30min |
| 8: 3rd | 25 | 60 | H+, VD+ | Ab− | Ster; BS | 27, C | PN death | 24h |
| 9: 3rd | 21 | 70 | No | Ab+ | Ster | 35, C | PM+ | 36min |
| 10: 3rd | 26 | 50 | No | Ab+ | Ster; BS | 38, C | PM+ | 2min |
| 11: 3rd | 20 | 60 | No | Ab+ | Ster | 36, C | PM− | 16min |
| 12: 3rd | 31 | 49 | No | Ab+ | No | 37, C | PM− | 12min |
| 13: 3rd | 20 | 50 | H+ | Ab+ | Ster | – | VTP, 22 WGA | – |
| 14: 3rd | 22 | 55 | No | Ab+ | No | – | VTP 23 WGA | – |
| 15: 3rd | 22 | 60 | No | Ab + | No | 39, C | PM− | 17min |
| 16: 3rd | 23 | 50 | VD+ | Ab+ | Ster | 37, V | IU death | – |
| 17: 3rd | 26 | 65 | H+, VD+ | Cmp+;Ab+ | Ster | 33, C | PM+ | 12min |
| 18: 3rd | 27 | 55 | H+, VD+ | Cmp+;Ab+ | Ster | 33, C | PM+, HT+ | – |
| 19: 3rd | 25 | 53 | H+, VD+ | Cmp+;Ab+ | Ster | 36, C | PM+ | 12min |
| 20: 3rd | 14 | – | No | Isom., CHD | No | – | VTP, 14 WGA | – |
| 21: 3rd | 23 | 63 | H+, VD+ | Isom.,CHD | No | – | IU death | – |
| 22: 3rd | 15 | 35 | H+, VD+ | Isom.,CHD | No | – | VTP, 16 WGA | – |
Ab: maternal antibodies (+, positive; −, negative); AVB: atrioventricular block; BS: beta-stimulants; C: caesarean section; CHD: congenital heart disease; Cmp: cardiomyopathy; Deg: degree; DFU: duration of postpartum follow-up; Dx: diagnosis; HT: heart transplant; Immunol.: immunology; Isom: isomerism; IU: intrauterine; PM: pacemaker (+, pacemaker implantation; –, no pacemaker implantation in an average of 18 months); PN: postnatal; Ster: steroids; Tx: treatment; V: vaginal; VD: ventricular dysfunction; VR: ventricular rate; VTP: voluntary termination of pregnancy; WGA: weeks of gestational age.
Characteristics and outcomes of foetuses with atrioventricular block.
| Characteristics and outcomes of 19 foetuses with high-degree AVB | ||
| 2nd degree AVB | 2 | 10% |
| 3rd degree AVB | 17 | 90% |
| Ab−/Ab+/CHD | 4/12/3 | 21%/63%/16% |
| Overall mortality | 3 spo. in 15 (+4 VTP/19) | 20% |
| CHD mortality | 1 spo. + 2 VTP in 3 CHD cases | |
| Ac− mortality | 1 of 4 | 25% |
| Ac+ mortality (VTP) | 1 of 10 (2 VTP in 12 cases) | 10% (2 VTP in 12 cases) |
| Pacemaker: 2nd and 3rd degree AVB | 7 of 12 surviving | 58% |
| Characteristics and outcomes of 2 foetuses with 2nd degree AVB | ||
| Ab−/Ab+/CHD | 1/1/0 | |
| Mortality | 0 | 0 |
| Tx with steroids | 1 (Ab+) | Prenatal regression to sinus rhythm, postnatal 3rd degree AVB, PM+ |
| PM+ | 1 | |
| Characteristics and outcomes of 17 foetuses with 3rd degree AVB | ||
| Ac−/Ac+/CC | 3/11/3 | 17.5%/65%/17.5% |
| Total mortality | 3 spo. deaths in 13 (+ 4 VTP/17 foetus in total) | 23% (+ 4 VTP/17) |
| Tx with steroids | 9 (8 AC+ and 1 Ac−) | |
| Beta-stimulants | 2 | |
| Characteristics and outcomes of 3 foetuses with 3rd degree AVB and Ac− | ||
| H+VD | 1 of 3 | 33% |
| Mortality | 1 of 3 (1 de 1: H+VD) | 33% |
| Pacemaker | 1 of 2 surviving | 50% |
| Tx with steroids | 1 (with H+VD) of 3 | 33% |
| Estimated efficacy | 0 of 1 | 0% |
| Characteristics and outcomes of 11 foetuses with 3rd degree AVB and Ab+ | ||
| VD (VD+H) | 4 (3) | 36% (27%) |
| Total mortality | 3 of 11 | 27% |
| Spontaneous mortality | 1 of 9 | 11% |
| Tx with steroids | 8 of 11 | 73% |
| regression of 3rd degree BAV | 0 of 8 | 0% |
| Hydrops improvement with tx | 2 of 8 (1 of 2 died) | 25% |
| Pacemakers/live births | 5 of 8 | 62% |
| Heart transplant | 1 of 3 with VD born alive | 33% |
| Mean FU | 18 months | |
| Characteristics and outcomes of 3 foetuses with 3rd degree AVB and CHD (all 3 with isomerism) | ||
| Spo. death +VTP | 1 spo.+2 VTP of 3 foetuses | 33.3%+66.6% |
| H + VD | 2 | 66% |
| Tx with steroids | 0 | 0% |
Ab: antibodies; AVB: atrioventricular block; CHD: congenital heart disease; FU: postpartum follow-up; H: hydrops; MC: maternal antibodies (+ positive; −, negative); PM: pacemaker (+, pacemaker implantation; –, no pacemaker implantation in 2 years of follow-up); spo: spontaneous; tx: treatment; VD: ventricular dysfunction; VTP: voluntary termination of pregnancy.
The management of AVBs varied depending on the hospital of diagnosis. While most facilities prescribed steroids to all foetuses with immune-mediated AVB, some facilities did not administer steroids in any of these cases. Steroid treatment was prescribed in 10 cases: the single case of immune-mediated second-degree AVB in this series, 8 out of the 11 cases of third-degree AVB positive for antibodies (73%), and 1 case negative for antibodies but with associated hydrops and VD. The case of second-degree AVB treated with steroids reverted to normal sinus rhythm before birth, but progressed to complete AVB after birth, requiring implantation of a pacemaker. Regression was not observed in any of the cases of third-degree AVB. There was some improvement in the signs of third-degree AVB in 2 of the 6 cases associated to hydrops and VD, but one of them resulted in death. Ten cases of AVB were treated with steroids; severe oligohydramnios developed in 1, and treatment was discontinued in another due to severe metrorrhagia. The steroid used in all cases was dexamethasone, administered in doses of 4mg every 24h; a loading dose of 6–8mg/day was administered in 3 cases. The mean duration of treatment was 5 weeks (range, 2–12 weeks), and in most cases treatment was discontinued a few weeks before birth. Two cases were treated with beta-stimulants; the treatment was ineffective in both, and had to be discontinued in 1 due to tachycardia and tremors in the mother. The predictors for poor outcome in foetuses with third-degree AVB in the absence of CHD were VD and hydrops; none of the foetuses without VD and hydrops died (0 of 7), while 2 of the 5 foetuses with hydrops and/or VD died (P=.15). One of the 3 foetuses with VD that survived needed a heart transplant later on. As for the heart rate of foetuses with no CHD, mortality in those with a ventricular rate of 55 beats per minute and more was 12.5% (1 in 8), compared to 25% (1 in 4) in foetuses with less than 55 beats per minute. During follow-up, which lasted a mean of 18 months postpartum (range, 24h–39 months postpartum), pacemakers were implanted in 58% of patients born alive with a prenatal diagnosis of high-degree AVBs.
DiscussionSustained foetal bradycardia always requires exhaustive investigation. If the bradycardia is caused by BPACs, the prognosis is good and it usually resolves spontaneously.18 Our series corroborated that foetuses with BPACs may develop sustained supraventricular tachycardia before or after birth, a complication that has been described in up to 13% cases in the literature.19 When it came to sinus bradycardia, which is commonly associated to sinus node dysfunction and a favourable prognosis,20 our series demonstrated that it can be associated to severe pathologies and a poor outcome. This finding is consistent with other studies that have shown an association of SB with intrauterine hypoxia and severe diseases, such as long QT syndrome21,22 and noncompaction cardiomyopathy.23 We want to highlight that anti-Ro and anti-La antibodies were found in a foetus diagnosed prenatally with SB and with sick sinus syndrome after birth, which shows that the range of diseases associated to those antibodies is quite broad.24
Our results corroborated that high-degree AVB is the most frequent cause of sustained foetal bradycardia in utero.2 For the purposes of analysis, this group of foetuses can be divided into 3 subsets according to aetiology: AVB secondary to cardiac malformation, AVB mediated by circulating maternal antibodies, and idiopathic AVB. Our case series confirms that the subset with AVB due to CHD has a very poor prognosis,25,26 and that the diagnosis of complete AVB is made earlier in association with this aetiology than with others (mean of 18 vs 23.8 WGA). In fact, first-trimester foetal bradycardia is considered a possible marker of left isomerism.27
Mortality in the idiopathic AVB subset of our study was 33%. Although prior research has shown that this subset has better outcomes,28 other studies have not confirmed this finding,14,15 and the number of cases in our study is too small to reach a reliable conclusion.
When it came to immune-mediated AVB, our case series was consistent with the time of prenatal diagnosis between 18 and 24 WGA described in the literature.3,4 We also found that mortality was considerable, even if it was lower than in cases associated with CHD. The predictors for poor outcome found in our series were consistent with those described in the literature: hydrops and VD at the time of diagnosis.3–5,15 When we analysed mortality in relation to a heart rate lower than 50–55 beats per minute, another predictor of poor outcome reported in previous case series,5,15 we found that a greater proportion of foetuses in our study died when the ventricular rate was lower than 55 beats per minute. This difference was not statistically significant, probably due to the sample size. Morbidity in cases of AVB diagnosed before birth was also high.3 Most of these patients required a pacemaker in the first 18 months of life, and one of them, who had VD, needed a heart transplant. The therapeutic approach of Spanish hospitals was heterogeneous as it concerned treatment of AVB with steroids, reflecting the current controversy that surrounds the subject. From a pathophysiological standpoint, steroids are administered in immune-mediated AVB to revert or diminish the inflammation that is part of the process leading to fibrosis of the conduction system.29 Many research groups have suggested courses of dexamethasone or betamethasone (fluorinated steroids that are not metabolised in the placenta), but their use is currently under debate.14,15 On one hand there is no strong evidence on the efficacy of this treatment, and a recent European retrospective multicentre study did not find statistically significant differences in foetal or neonatal mortality between treated and untreated foetuses.15 As for third-degree AVB, our series confirms that once the block is complete it is irreversible, a fact that is unquestioned in the scientific community.3,29 There are authors who suggest that the efficacy of steroids in reducing mortality can be demonstrated, particularly in cases with VD and hydrops.3,30,31 Our results did not support this approach. While 2 out of 5 foetuses that met this description showed signs of improvement, the latter was temporary and 1 of the foetuses died in utero. Treatment with steroids in foetuses with incomplete AVB is meant to prevent disease progression,29 and the efficacy of treatment in this set of patients is also subject to debate. It is not known whether first-degree AVB is the precursor of higher degrees of AVB, in which case treating it may be beneficial,32 or whether it results from physiological variations in vagal tone, in which case it would tend to revert spontaneously.33 Our series did not include any cases of suspected first-degree AVB with observation of prolonged atrioventricular conduction, and while there is no consensus on the management of these patients, we believe that steroid therapy should be offered, properly informing the parents about its risks and benefits, as this is the clinical presentation in which steroids are most likely to succeed. Cases of second-degree AVB that have regressed with steroid treatment have been reported,15,34 although frequently the regression was temporary and there was subsequent progression to third degree,15,34,35 as seen in the only case of second-degree AVB in our series; therefore, it is not clear that treatment is beneficial in these patients.14 While the therapeutic efficacy of steroids is still to be demonstrated, their prolonged use has been associated to serious adverse effects,15,36–38 such as intrauterine growth restriction, oligohydramnios (diagnosed in 10% of foetuses treated with steroids in our series), adrenal failure, and abnormal brain development in foetuses; and hypertension and gestational diabetes in pregnant women. In short, our data confirm that despite multiple international studies performed to this day, many doubts remain regarding the potential benefits of steroids in immune-mediated AVB,6,15 and consequently steroids should be used with extreme caution, especially considering their potential side effects.
LimitationsThere are limitations to this study. Although it was a multicentre study, this pathology is rare and the number of documented cases is small. Secondly, we were unable to analyse the relationship between the development of immune-mediated AVB and the amount of circulating antibodies, when previous case series found an association between the risk of foetal arrhythmias and higher levels of circulating antibodies.39 Thirdly, the postnatal follow-up was of short duration. Finally, since the study did not include all the foetal cardiology and prenatal diagnosis facilities in Spain, the results may not be fully representative of how foetal bradycardia is managed in Spain.
ConclusionsSustained foetal bradycardia always requires an exhaustive investigation, even in cases of sinus bradycardia. Bradycardia due to BPACs usually has a good prognosis, although there is a risk for tachyarrhythmias. Morbidity and mortality are considerable in high-degree AVB diagnosed in utero, especially in cases associated with CHD. Prenatal treatment of immune-mediated AVB remains controversial. Improved knowledge of the natural history of this disease could help develop new, more specific therapeutic strategies40 and select the foetuses that may benefit from them.
Conflicts of interestThe authors have no conflicts of interest to declare.
We want to thank Dolores Rubio, from the Hospital Universitario La Paz of Madrid, and Rosa Maria Perich, from the Hospital Universitario de Sabadell, Barcelona, for their valuable participation in the study.
Please cite this article as: Perin F, Rodríguez Vázquez del Rey MM, Deiros Bronte L, Ferrer Menduiña Q, Rueda Nuñez F, Zabala Arguelles JI, et al. Bradicardia fetal: estudio multicéntrico retrospectivo en 9 hospitales españoles. An Pediatr (Barc). 2014;81:275–282.
Previous presentation: This study was presented at the 45th Annual Meeting of the Association for European Paediatric and Cardiology (AEPC); May 20, 2011; Granada, Spain.