There is no consensus on which neonatal chart is best to use in very low birth weight (VLBW) infants. The aim of the study was to compare the Fenton 2013 and Intergrowth-21st (IW-21) charts based on their predictive ability for somatometry at 2 years, as well as to analyze factors related to short stature at 2 years.
Material and methodsCohort of children with VLBW born between 2002 and 2017. Association between neonatal somatometry (z-score by Fenton and IW-21) and risk of short stature (<−2 DS), head circumference <−2 DS and malnutrition at 2 years (BMI < −2 DS) was analyzed (WHO charts).
Results513 children with a mean gestational age of 30.05 ± 2.5 weeks were included. Birth and discharge weight z-score by Fenton and IW-21 were useful for predicting risk of short stature and malnutrition at 2 years (without differences in the AUC of the ROC curves). Weight z-score at discharge was also useful for predicting head circumference < −2 DS. At 2 years, prevalence of short stature, head circumference < −2 DS, and malnutrition was 17.2, 4.1, and 6.1%, respectively. Low weight for gestational age and length of stay were identified as independent risk factors for short stature at 2 years.
ConclusionsDischarge weight z-score is useful for predicting risk of short stature, malnutrition and head circumference < −2 DS at 2 years in very low birth weight children, with no statistical difference between using Fenton or IW-21 charts.
No existe consenso sobre qué gráfica neonatal es mejor utilizar en niños con muy bajo peso al nacer (MBPN). El objetivo del estudio fue comparar las gráficas de Fenton 2013 e Intergrowth-21st (IW-21) con base en su capacidad predictora de la somatometría a los dos años, así como analizar factores relacionados con talla baja a los dos años.
Material y métodosCohorte de niños con MBPN nacidos entre 2002−2017. Se analizó la asociación entre la somatometría neonatal (z-score por Fenton e IW-21) y el riesgo de talla baja (<−2 desviación estándar [DS]), perímetro craneal < −2 DS y desnutrición a los 2 años (IMC < −2 DS) (gráficas OMS).
ResultadosSe incluyeron 513 niños con una edad gestacional media de 30,05 ± 2,5 semanas. El z-score del peso al nacimiento y al alta por Fenton y por IW-21 resultaron útiles para predecir riesgo de talla baja y desnutrición a los dos años (sin diferencias en el AUC de las curvas ROC), siendo el z-score al alta útil además para predecir perímetro craneal < −2D. A los dos años, la prevalencia de talla baja, perímetro craneal < −2 DS, y desnutrición fue del 17,2, 4,1 y 6,1%, respectivamente. El bajo peso para la edad gestacional y la duración del ingreso neonatal se identificaron como factores de riesgo independientes para talla baja a los dos años.
ConclusionesEl z-score peso al alta resulta útil para predecir riesgo de talla baja, desnutrición y perímetro craneal < −2 DS a los dos años en niños con muy bajo peso al nacer, sin diferencias estadísticas entre utilizar las gráficas de Fenton o IW-21.
At present, very low birth weight (VLBW) infants constitute a significant public health problem. In our society, advanced maternal age pregnancies, pregnancies in mothers with chronic disease or multiple pregnancies following assisted reproductive techniques, all of them factors that contribute to the birth of infants with VLBW, are increasingly frequent.1–4 Although perinatal mortality is decreasing progressively in preterm infants, morbidity continues to be high.5,6 The growth of VLBW preterm infants is characterised by a period of growth restriction in the immediate postpartum period followed by a period of catch-up growth that has the most impact in the first 2–3 years. Usually, this catch-up growth is incomplete and preterm infants grow to have shorter stature and lower weights in adulthood compared to their full-term peers, in addition to abnormalities in body composition associated with an increased cardiovascular risk.7–12 At discharge from hospital, VLBW infants are usually at lower percentiles than expected based on intrauterine growth references, usually below the 10th percentile (P10).13 In Spain, the prevalence of small for gestational age (SGA) in VLBW infants is 27%, and 77% of these infants are discharged with weights below the P10.14
The classification of postnatal growth in children with VLBW can be made using intrauterine or extrauterine growth charts. Historically, since 1977, the American Academy of Pediatrics has recommended using intrauterine growth charts as reference for the extrauterine growth of preterm infants, and the most widely used charts for the purpose are those published by Fenton, although preterm infants rarely meet the targets set by foetal growth charts.15,16 The Fenton growth charts, updated in 2013, are based on foetal somatometric measurements at birth for each gestational age and by sex. They were generated with the data of nearly 4 million preterm infants born in Germany, the United States, Italy, Australia, Scotland and Canada, and from 50 weeks of postmenstrual age, the assessment of growth continues using the growth standards of the World Health Organization (WHO).17
In recent years, some authors have proposed the use of growth standards based on the longitudinal growth of healthy preterm infants.18 This led to the publication of the reference data of the INTERGROWTH-21st (IW-21) study, including charts for assessment of neonatal anthropometric measurements and of postnatal growth. The charts were developed through the prospective collection of data between 2009 and 2014 of 4607 children, of who only 224 (5%) had been born preterm, applying the following criteria: pregnancies in healthy women that conceived naturally, who received antenatal care in the context of which there was no evidence of intrauterine growth restriction, with reliable estimation of gestational age from the first trimester, standardised measurements of the infant taken from birth and implementation of a nutritional protocol derived from presently recommended guidelines.19 The IW-21 standards were developed for universal application, and including patients from 8 countries (Brazil, Italy, Oman, United Kingdom, United States, China, India and Kenia) and overlap the WHO growth standards from 64 weeks of postmenstrual age.18,19 The WHO recommends using the IW-21 and WHO growth standards to guide the assessment of growth in children born preterm.20
The greatest change in weight gain takes place in the first 2 years of life, when children can exhibit more substantial catch-up growth. From this age, the growth pattern is usually determined by genetics. Early catch-up growth is associated with an increased risk of obesity in childhood and possibly of disease in adulthood.21 Postnatal anthropometry is an important parameter in children born preterm to assess the risk of unfavourable long-term outcomes. Correct classification of these children is important not only to assess their growth, but also to establish an appropriate post-discharge nutrition plan.
Currently, there is no consensus as to which reference is best for assessing neonatal growth and which is best to identify children at risk of unfavourable growth outcomes at age 2 years. Theoretically, the IW-21 growth standards, based on prospective data on preterm infants with minimum morbidity, should fit the growth of preterm infants better than the Fenton charts, which are based on intrauterine growth. The aim of our study was to compare the use of the 2013 Fenton charts and the IW-21 growth standards in the neonatal period in infants with birth weights of less than 1500 g in relation to the risk of short stature, undernutrition, a head circumference z-score (HCz) of less than –2 and obesity at 2 years of corrected age, and to identify factors associated with short stature at 2 years.
Material and methodsWe conducted a retrospective and descriptive study in a cohort that included all infants with a birth weight of less than 1500 g born between 2002 and 2017 and managed in the neonatal unit of the Hospital Universitario Central de Asturias (HUCA) in Oviedo, Spain. All such infants were included in the perinatal morbidity database of the Very Low Birth Weight Infant Network (SEN1500) register and the 2-year follow-up after the parents provided signed informed consent.22 The HUCA is a tertiary care hospital with a catchment population of 1 million inhabitants that manages approximately 5000 births a year. The exclusion criteria were: gestational age of less than 24 weeks, death, presence of major congenital anomalies, chromosomal disorders, congenital disease associated with or lack of follow-up in outpatient clinics through 24 ± 6 months of corrected age (the discharge plan of all patients included outpatient follow-up).
We calculated weight, length and head circumference z-scores at birth and at discharge using the Fenton and IW-21 charts. We defined SGA as a birth weight below the P10, and classified infants above that percentile as appropriate for gestational age. We calculated the body mass index (BMI) from the weight and length with the formula [weight in g]/[length in cm]2 * 10, after which we calculated the BMI z-score using the Olsen curves as reference.23
At 2 years of corrected age, we calculated the weight, height, head circumference and BMI z-scores using the WHO growth standards.24 We calculated the BMI as [weight in kg]/[height in m]2. At age 2 years, we defined short stature, low weight and undernutrition as height, weight and BMI z-scores of less than –2, respectively, and obesity as a BMI z-score greater than 2.25,26
Statistical analysisThe statistical analysis was performed with the software SPSS version 24.0 (IBM Corp, Armonk, NY, USA). We have described qualitative variables as absolute frequencies and percentages, and quantitative variables as mean and standard deviation (SD) or as median and interquartile range (IQR). We compared qualitative variables with the χ2 test and quantitative variables with the Student t test or Mann-Whitney U test as applicable. Interrater agreement was assessed with the kappa statistic. We generated receiver operating characteristics (ROC) curves to estimate the power of the weight and the length z-scores at birth and at discharge to predict short stature, undernutrition, a HCz of less than –2 and obesity at 2 years.
We compared the areas under the curve (AUCs) of the ROC curves with the software Epidat 3.1 (free access software, Department of Health of Galicia and Pan American Health Organization [PAHO-WHO]) using the Delong test27 or empirical nonparametric method. With these approaches, assuming a standard deviation of 0.01 and an alpha level of 0.05, it would be possible to detect differences of 0.025 with a power of 80%.
To assess correlation, we calculated Pearson or Spearman correlation coefficients.
We fit a multivariate logistic regression model for the risk of short stature at 2 years using a stepwise approach with forward selection of variables that had been found to be significantly associated in the univariate analysis.
We defined statistical significance as a P value of less than 0.05.
The study was approved by the Research Ethics Committee (file no. 2020.314).
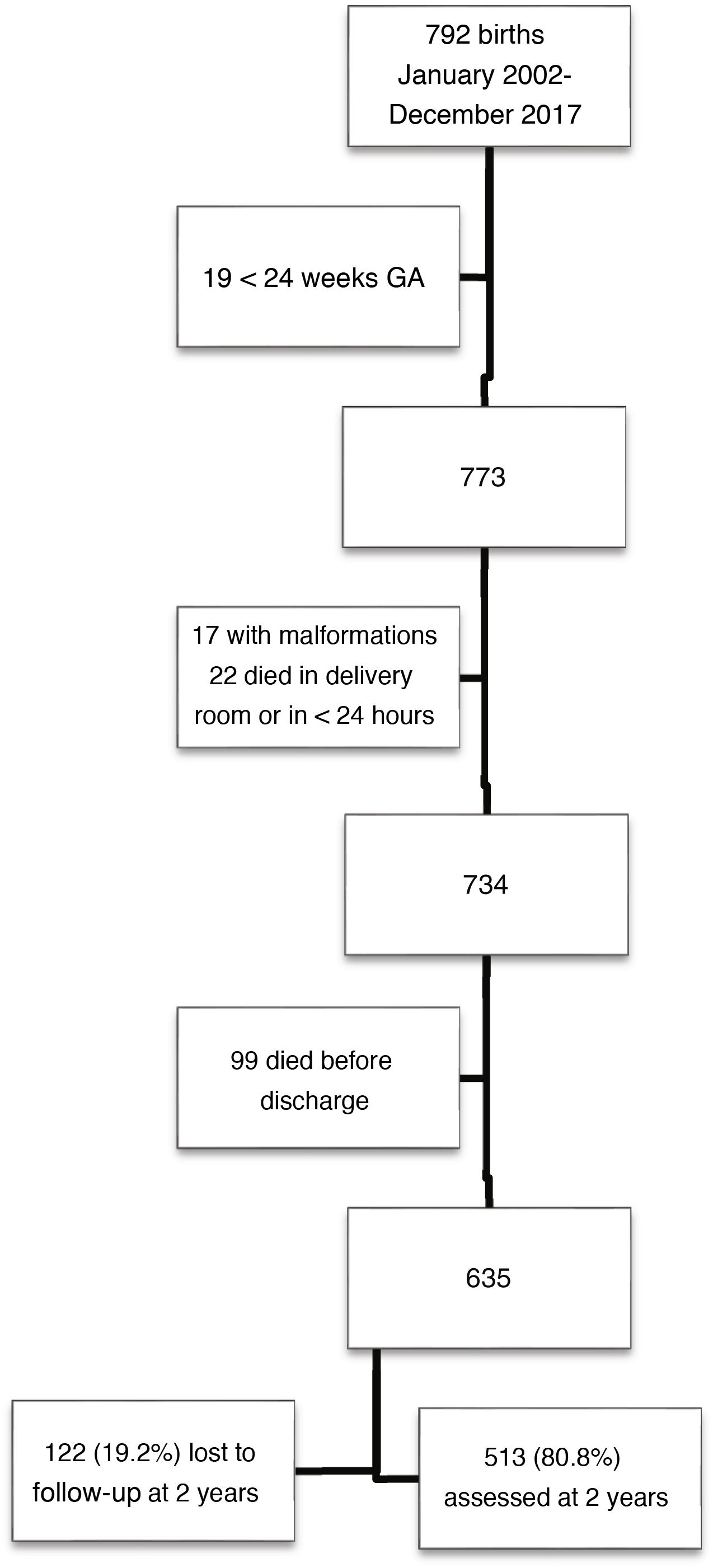
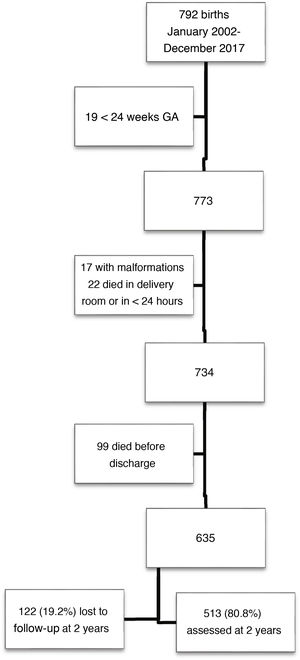
ResultsOf a total of 792 children born with VLBW, 513 met the inclusion criteria and completed follow-up through 2 years of chronological age. Fig. 1 presents a flow chart of patient selection.
Children in the sample were born at a mean of 30.0 weeks of gestation (SD, 2.5), with a mean birth weight of 1155 g (SD, 244). The percentage born SGA as 36.8% based on the Fenton charts and 35.3% based on the IW-21 standards (kappa = 0.882). The prevalence of SGA did not vary significant through the study period, which we divided into two 8-year periods (Fenton: 35.5% vs 38.2% [P = 0.53]; IW-21: 34.4% vs 36.2% [P = 0.66]). Table 1 presents the characteristics of the sample and the morbidity in the neonatal period.
Characteristics of the study cohort, comparing patients that completed the follow-up versus those who were lost to follow-up at 2 years of corrected age.
| Complete FU, n = 513 | Lost to FU, n = 122 | P | |
|---|---|---|---|
| Gestational age | |||
| < 28 weeks | 108 (21.1%) | 15 (12.3%) | 0.028 |
| At discharge (weeks) | 38.7 ± 2.6 | 0.147 | |
| Perinatal characteristics | |||
| Male sex | 249 (48.5%) | 57 (46.7%) | 0.718 |
| Antenatal steroids (full course) | 296 (58.3%) | ||
| Multiple gestation | 160 (31.2%) | ||
| Caesarean delivery | 379 (73.9%) | ||
| 5-min Apgar < 5 | 16 (3.1%) | ||
| SGA (Fenton 2013) | 189 (36.8%) | 43 (35.2%) | 0.742 |
| SGA (INTERGROWTH-21 st) | 181 (35.3%) | 42 (34.4%) | 0.859 |
| Neonatal morbidity | |||
| Hyaline membrane disease | 254 (49.5%) | ||
| Mechanical ventilation | 285 (55.6%) | ||
| Sepsis < 72 h post birth | 18 (3.5%) | ||
| Sepsis > 72 h post birth | 167 (32.6%) | ||
| Parenteral nutrition at 28 days post birth | 46 (9) | ||
| Necrotising enterocolitis | 16 (3.1%) | ||
| Patent ductus arteriosus | 127 (24.8%) | ||
| Anaemia (transfusion) | 145 (34.9%) | ||
| Acute kidney injury | 11 (2.7%) | ||
| Hypotension (inotropes) | 41 (8%) | ||
| Retinopathy of prematurity stage ≥ 2 | 54 (11.7%) | ||
| Bronchopulmonary dysplasia | 104 (20.3%) | ||
| Periventricular leukomalacia | 52 (10.1%) | ||
| IVH grade 3−4 | 22 (4.3%) | ||
| Length of stay | 54 (43−74) | 50 (38−66) | 0.047 |
| Somatometry at birth | |||
| Weight (g) | 1155 ± 244 | 1248 ± 204 | <0.0001 |
| Length (cm) | 37.9 ± 3.1 | 38.8 ± 2.7 | 0.04 |
| Head circumference (cm) | 26.4 ± 2.2 | 27.0 ± 1.8 | 0.001 |
| BMI (g/cm2) | 7.90 ± 0.87 | 8.25 ± 1 | <0.0001 |
| Somatometry at discharge | |||
| Weight (g) | 2400 ± 285 | 2484 ± 380 | 0.023 |
| Length (cm) | 45.5 ± 2.1 | 46.2 ± 2.3 | 0.01 |
| Head circumference (cm) | 33.2 ± 1.5 | 33.3 ± 1.6 | 0.632 |
| BMI (g/cm2) | 11.55 ± 1.09 | 11.62 ± 1.12 | 0.499 |
| EUGR (Fenton 2013) | 374 (73%) | 94 (77%) | 0.366 |
| EUGR (INTERGROWTH-21st) | 284 (55.4%) | 59 (48.4%) | 0.16 |
Values expressed as absolute frequency (percentage), mean ± standard deviation or median (interquartile range).
BMI, body mass index; EUGR, extrauterine growth restriction (discharge weight < 10th percentile); FU, follow-up; IVH, intraventricular haemorrhage; SGA, small for gestational age.
Statistical analysis: χ2, Student t test and Mann-Whitney U test.
At 2 years of corrected age, we found short stature in 17.2% of the sample, a HCz of less than –2 in 4%, undernutrition in 6% and obesity in 2.5% (Table 2).
Classification of somatometric measurements at 2 years of corrected age based on the height, weight, head circumference and BMI in children with birth weights of less than 1500 g.
| n | % | |
|---|---|---|
| Height z-score at 2 years, n = 512 | ||
| ≤–2 | 88 | 17.2% |
| z-score: mean ± SD | –0.88 ± 1.19 | |
| Weight z-score at 2 years, n = 513 | ||
| ≤–2 | 71 | 13.8% |
| z-score: mean ± SD | –0.68 ± 1.18 | |
| HC z-score at 2 years, n = 510 | ||
| ≤–2 | 21 | 4.1% |
| z-score: mean ± SD | 0.28 ± 1.26 | |
| BMI z-score at 2 years n = 512 | ||
| ≤–2 | 31 | 6.1% |
| –2 to 1 | 412 | 80.5% |
| 1 to 2 | 57 | 11.1% |
| >2 | 13 | 2.5% |
| z-score: mean ± SD | –0.17 ± 1.18 | |
BMI, body mass index; HC, head circumference; SD, standard deviation.
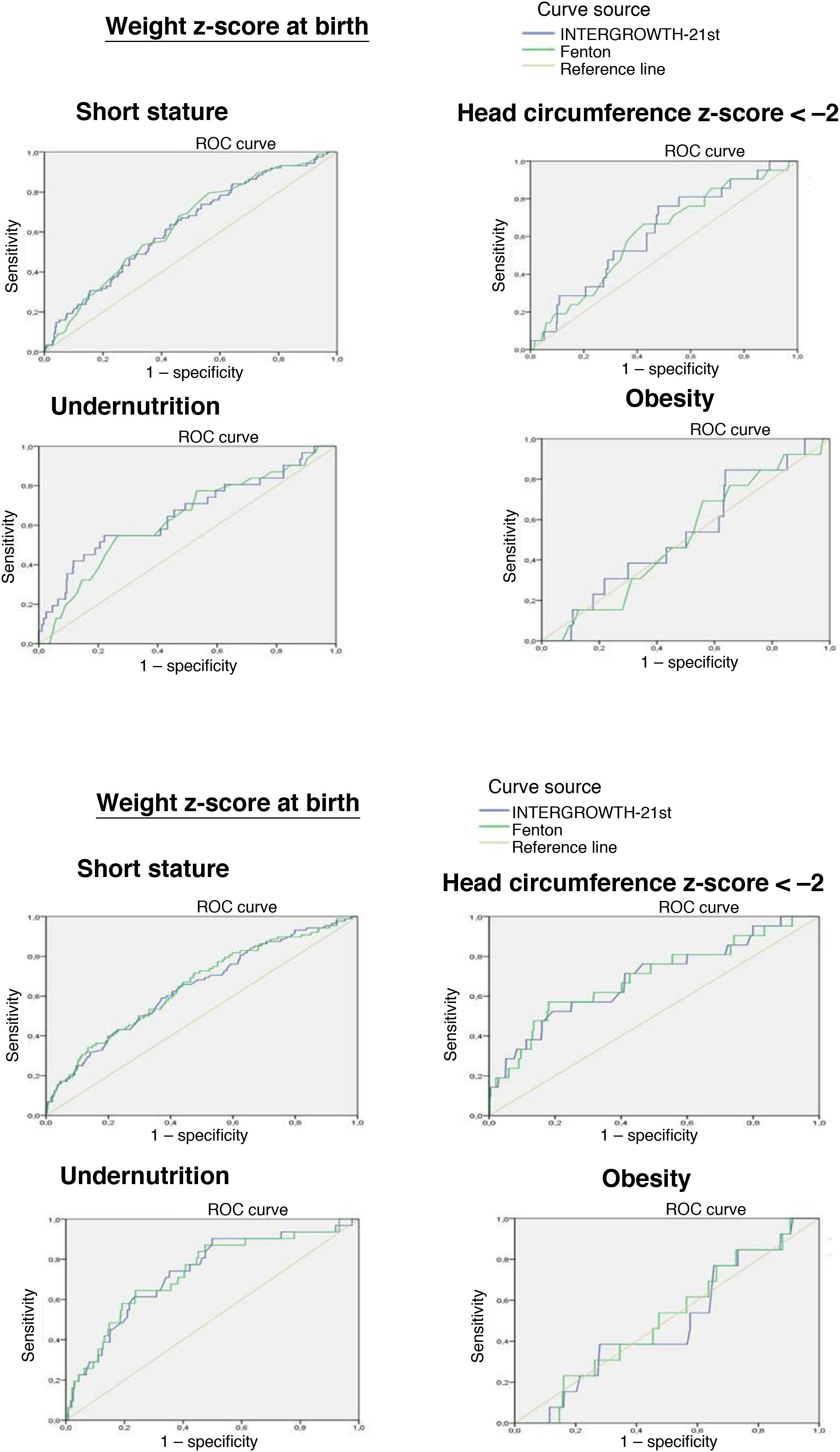
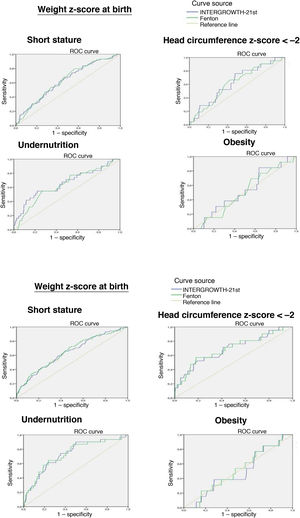
The ROC curve showed that the birth weight z-score was associated with an increased risk of short stature at 2 years (IW-21: AUC = 0.629, 95% confidence interval [CI], 95% 0.566−0.692, P < 0.0001; Fenton: AUC = 0.634, 95% CI, 0.573−0.695, P < 0.0001) and an increased risk of undernutrition at 2 years (IW-21: AUC = 0.657, 95% CI, 0.546−0.768, P = 0.003; Fenton: AUC = 0.634, 95% CI, 0.531−0.737, P = 0.012). However, it was not associated with an increased risk of a HCz of less than –2 (IW-21: AUC = 0.625, 95% CI, 0.510−0.739, P = 0.053; Fenton: AUC = 0.613, 95% CI, 0.496−0.713, P = 0.079).
The weight z-score at discharge was associated with an increased risk of short stature (IW-21: AUC = 0.650, 95% CI, 0.587−0.71, P < 0.0001; Fenton: AUC = 0.643, 95% CI, 0.579−0.714, P < 0.0001), HCz of less than –2 (IW-21: AUC = 0.693, 95% CI, 0.567−0.820, P = 0.003; Fenton: AUC = 0.690, 95% CI, 0.564−0.814, P = 0.003) and malnutrition (IW-21 AUC: 0.732, 95% CI, 0.639−0.825, P < 0.0001; Fenton: AUC = 0.732, 95% CI, 0.640−0.824, P < 0.0001) at 2 years.
Comparing the AUCs for the ROC curves, we did not find statistically significant differences between the use of the Fenton charts or IW-21 standards at birth or at discharge.
We did not find an association between the weight z-score and the risk of obesity at 2 years with either the Fenton or the IW-21 reference (Fig. 2).
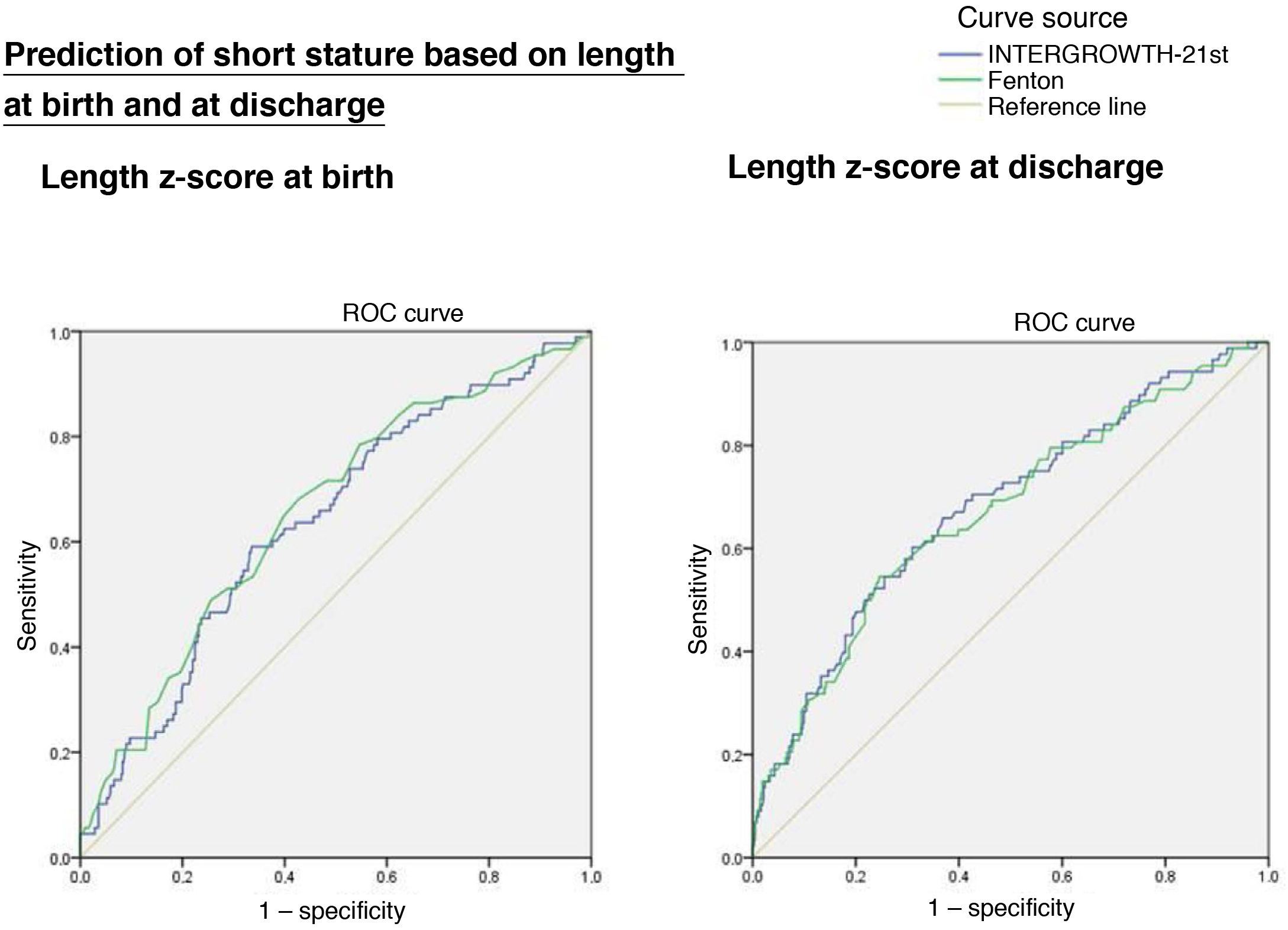
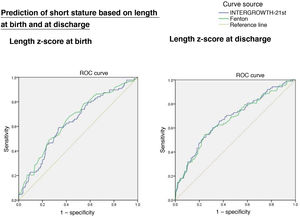
Length z-scoreThe ROC curves showed an association between short stature at age 2 years with the length z-score at birth (IW-21: AUC = 0.636, 95% CI, 0.573−0.699, P < 0.0001; Fenton: AUC = 0.655, 95% CI, 0.592−0.717, P < 0.0001) and at discharge (IW-21: AUC = 0.679, 95% CI, 0.616−0.742, P < 0.0001; Fenton: AUC = 0.668, 95% CI, 0.604−0.32, P < 0.0001), without statistically significant differences between the AUCs of the Fenton and the IW-21 ROC curves (Fig. 3).
Factors with an impact on height at age 2 yearsTable 3 presents the correlation coefficients and P values obtained in the comparison of somatometric values obtained at birth and discharge using the Fenton and IW-21 references with height outcomes at 2 years.
Factors associated with the height z-score at 2 years of corrected age in infants with birth weights of less than 1500 g (correlation coefficient).
| Correlation coefficient (r) | P | |
|---|---|---|
| Gestational age (weeks) | –0.04 | 0.32 |
| Somatometry at birth | ||
| IW-21 weight z-score | 0.23 | <0.0001 |
| Fenton weight z-score | 0.24 | <0.0001 |
| IW-21 length z-score | 0.28 | <0.0001 |
| Fenton length z-score | 0.294 | <0.0001 |
| Somatometry at discharge from neonatal unit | ||
| IW-21 weight z-score | 0.264 | <0.0001 |
| Fenton weight z-score | 0.273 | <0.0001 |
| IW-21 length z-score | 0.354 | <0.0001 |
| Fenton length z-score | 0.354 | <0.0001 |
IW-21, INTERGROWTH growth standards; Fenton, 2013 Fenton growth charts.
When it came to the perinatal characteristics of children with short stature at age 2 years, we observed a higher frequency of birth weight less than 1000 g (44% vs 25%; P < 0.0001), SGA (IW-21: 48.9% vs 32.5% [P = 0.004]; Fenton: 53.4% vs 33.5%; P < 0.0001) and hypotension (14.8% vs 6.6%, P = 0.01). Patients with short stature at age 2 years were less likely to have a history of hyaline membrane disease, yet there were no differences between patients with and without short stature in the use of mechanical ventilation or its duration or the use of oxygen therapy (Table 4).
Perinatal characteristics of patients with short stature at 2 years of corrected age compared to patients born with VLBW with a normal height.
| Short stature, n = 88 | Normal height, n = 424 | P | OR (95% CI) | aOR (95% CI) | P | |
|---|---|---|---|---|---|---|
| Male sex | 40 (50%) | 204 (48.1%) | 0.747 | |||
| Gestational age | 30.1 ± 2.6 | 30.0 ± 2.5 | 0.759 | |||
| Gestational age < 28 weeks | 18 (20.5%) | 90 (21.2%) | 0.872 | |||
| Birth weight < 1000 g | 39 (44.3%) | 106 (25%) | <0.0001 | 2.38 (1.48−3.83) | 1.87 (0.97−3.59) | 0.059 |
| SGA (INTERGROWTH-21st) | 43 (48.9%) | 138 (32.5%) | 0.004 | 1.98 (1.24−3.15) | 1.69 (1.00−2.84) | 0.047 |
| SGA (Fenton 2013) | 47 (53.4%) | 142 (33.5%) | <0.0001 | 2.27 (1.43−3.62) | ||
| 5-min Apgar < 5 | 3 (3.4%) | 13 (3.1%) | 0.745 | |||
| Hyaline membrane disease | 35 (39.8%) | 218 (51.4%) | 0.047 | 0.624 (0.391−0.996) | 0.40 (0.22−0.72) | 0.002 |
| Mechanical ventilation | 45 (51.1%) | 241 (56.8%) | 0.327 | |||
| Duration of mechanical ventilation (h) | 10.5 (0−96) | 18 (0−120) | 0.747 | |||
| Duration of oxygen therapy (h) | 43 (2.2−230) | 50.5 (3−216) | 0.788 | |||
| Early-onset sepsis | 3 (3.4%) | 15 (3.5%) | 1.0 | |||
| Late-onset sepsis | 33 (37.5%) | 134 (31.6%) | 0.283 | |||
| Parenteral nutrition at 28 days post birth | 7 (8%) | 39 (9.2%) | 0.701 | |||
| Necrotising enterocolitis | 4 (4.5%) | 12 (2.8%) | 0.496 | |||
| Patent ductus arteriosus | 24 (27.3%) | 103 (24.3%) | 0.556 | |||
| Anaemia (transfusion) | 26 (41.9%) | 119 (33.8%) | 0.216 | |||
| Acute kidney injury | 3 (4.8%) | 8 (2.3%) | 0.220 | |||
| Hypotension (inotropes | 13 (14.8%) | 28 (6.6%) | 0.01 | 2.45 (1.21−4.94) | 2.08 (0.90−4.78) | 0.085 |
| Retinopathy of prematurity grade ≥ 2 | 11 (14.5%) | 43 (11.1%) | 0.408 | |||
| Bronchopulmonary dysplasia | 19 (21.6%) | 85 (20%) | 0.743 | |||
| Periventricular leukomalacia | 10 (11.4%) | 42 (9.9%) | 0.680 | |||
| Intraventricular haemorrhage grade 3−4 | 4 (4.5%) | 18 (4.2%) | 0.899 | |||
| Length of stay | 59 (46−88) | 53.5 (43−72) | 0.022 | 1.01 (1.00−1.02) | 0.047 |
Values expressed as absolute frequency (percentage), mean ± standard deviation or median (interquartile range).
aOR, adjusted odds ratio; OR, odds ratio; SGA, small for gestational age (birth weight < 10th percentile).
Statistical analysis: χ2, Fisher exact test, Student t test, Mann-Whitney U test and multivariate logistic regression.
In the logistic regression analysis of the risk of short stature, the variables found to be independently associated with short stature at 2 years were the total length of stay and SGA classification using the IW-21 standard. Hyaline membrane disease was identified as an independent protective factor (Table 4).
Children born SGA were more likely to have short stature and a lower BMI, weight, height and HC at age 2 years (Appendix B, supplemental table).
DiscussionIn our study, the weight z-scores at birth and discharge were useful to predict short stature and undernutrition at 2 years in VLBW infants, with no statistically significant differences when we compared the application of the Fenton and IW-21 references. The weight z-score at discharge, which was also useful to predict a HCz of less than –2, without significant differences based on the use of either reference. We did not find an association with the risk of obesity at age 2 years. Similarly, the length z-scores at birth and at discharge were useful for predicting short stature at age 2 years (without significant differences based on the use of the Fenton or IW-21 charts). Small for gestational age and a greater length of stay were independent risk factors for short stature at age 2 years.
Of the total sample, 82.8% of children reached heights within the normal range at 2 years of corrected age, although the mean was below the mean height for age. This was consistent with the findings of other case series and could be a risk factor for short stature in adulthood.
Lebrão et al28 compared the weight percentiles at birth calculated with the Fenton and IW-21 charts in preterm infants born between 26 and 33 weeks’ gestation and analysed their association with growth outcomes at 12 months. They found that the IW-21 was slightly superior to the Fenton in the prediction of overweight and short stature at 12 months of corrected age. In this case series, 33.5% of infants had short stature, 11% undernutrition and 7.5% a HCz of less than –2 at age 12 months. In contrast, in our study in a cohort of infants of similar gestational age and mean birth weight, we found lower proportions of short stature (17.2%), undernutrition (6%) and HCz of less than –2 (4%,) at 2 years. On the other hand, in a cohort of 322 infants with birth weights of less than 1500 g, Takayanagi et al.29 found a prevalence of short stature and undernutrition of 11.8% at age 6 years, and these outcomes were significantly associated with extrauterine growth restriction. All of these discrepancies are probably related to the methodological heterogeneity of the studies and the variability in the duration of catch-up growth.
The prevalence of short stature was significantly higher in infants born SGA (23.8% compared to 13.6%; P = 0.004), and SGA infants also had lower weights, BMIs and HCs at age 2 years. Although the prevalence of short stature was high, it was within what would be expected based on the findings in other cohorts. For example, the prevalence of short stature (height z-score < –2.5) was 17% in a cohort of 217 SGA infants with VLBW in a case series published by Arai et al. with a 3-year follow-up,30 and the prevalence in the study by Takayanagi et al.29 was 27%. Although there is variation in the duration of follow-up, the findings of these studies suggests that the probability of catch-up growth in SGA infants born preterm is lower compared to term infants (10%), which makes predicted adult height outcomes bleaker.30–33 Several studies have evinced that altered growth in early childhood, especially in the first 2 years of life, has an impact on adult height.34,35 We ought to mention the limitation inherent in the fixed definition of SGA, which encompasses both infants that are constitutionally small and infants with a history of intrauterine growth restriction.
We found a positive association between the weight and length z-scores at birth and the height z-score at age 2 years, which stronger associations with length and with somatometric measurements at discharge compared to at birth. Similarly, Kim et al36 found that birth length was more strongly correlated to height at 2 years than birth weight in a sample of 130 infants born before 37 weeks of gestational age.
In the univariate analysis, short stature at 2 years was significantly associated with birth weight of less than 1000 g, SGA, prolonged length of stay and low blood pressure. On the other hand, in the logistic regression analysis, a prolonged length of stay and SGA were associated with short stature at age 2 years. Our findings were consistent with those of Durá-Travé et al.,37 who followed up a cohort of 170 VLBW infants through age 14 years and found that a birth weight of less than 1000 g was independently associated with short stature at ages 2, 4 and 10 years. Similarly, a study conducted by Kim et al.36 found that an increased length of stay in the neonatal unit was an independent risk factor for poor height outcomes at age 2 years. This evidence suggests that failure of catch-up growth may be associated not only with somatometric measurements at birth, but also with other postnatal factors.
The Fenton charts are routinely used in clinical practice, but they are based on intrauterine foetal growth, as opposed to the IW-21 standards, which are based on the actual longitudinal growth of healthy preterm infants. However, despite the theoretical advantages of the IW-21 standards, they did not perform better in the prediction of short stature, undernutrition or a HCz of less than –2 at 2 years in our case series. We found good agreement (k = 0.88) between the Fenton and IW-21 charts in the classification as SGA (36.8% and 35.8%, respectively). On the other hand, the IW-21 standards turned out to be more stringent for the classification of postnatal growth restriction (weight at discharge < P10: IW-21, 55% vs Fenton, 73%).
Among the limitations of the study, we ought to mention its retrospective observational design and that the data were collected in a single centre. The period under study was broad, which entails a change in nutritional guidelines according to the changes in international recommendations. In addition, due to the retrospective nature of the study, we could not take into account parental heights. Nevertheless, we believe that the sample size was sufficient to draw relevant conclusions.
ConclusionThe weight z-score at discharge is useful to predict the risk of low stature, undernutrition and a HCz of less than –2 at age 2 years, without significant differences based on the use of the Fenton growth chart or the IW-21 preterm postnatal growth standards. Children born with VLBW tend to catch up to term children in 2 years. However, a significant proportion of VLBW infants, especially those born SGA, have a short stature at 2 years of corrected age. We identified SGA and the neonatal length of stay as independent risk factors for short stature at age 2 years.
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.