Pregestational type 1 diabetes (T1D) is a risk factor for maternal and foetal complications related to pregestational and gestational glycaemic control.1 Optimal control in the first trimester of pregnancy is associated with improved obstetric outcomes, a lower incidence of congenital malformations and a reduction in perinatal mortality.2,3 However, maintenance of strict glycaemic control throughout pregnancy is very difficult, as insulin requirements are continuously changing during this period.4
We conducted a retrospective study in pregnant women with pregestational T1D and their newborn infants in the 2010–2018 period on the occasion of the opening of the pregestational diabetes clinic in our hospital. We collected data on glycaemic control, obstetric and perinatal complications in both treatment groups, and weekly insulin requirements exclusively in the group treated with continuous subcutaneous insulin infusion (CSII).
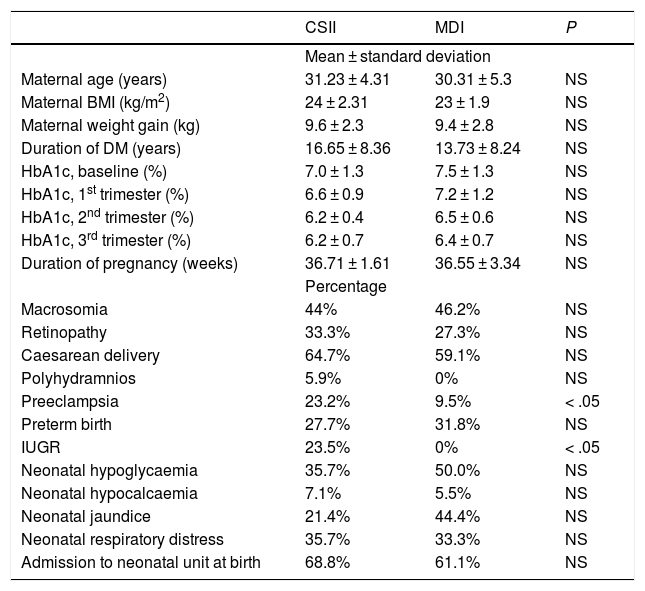
The sample included 39 pregnant women (18 treated with CSII, 21 with multiple dose injection [MDI] therapy). Of this total, 56.4% had received preconception care, and their glycaemic control was considered adequate for pregnancy based on the criteria established in the consensus guidelines of the Spanish Group on Diabetes and Gestation.5 We compared baseline characteristics and maternal and infant outcomes in both groups (Table 1). There were no instances of hospital admission due to acute decompensation secondary to T1D. The caesarean delivery rate was higher in both groups compared to the overall rate for our hospital (24.8%), and there was a significantly higher incidence of intrauterine growth restriction and preeclampsia in the CSII group. We did not find any other significant differences in metabolic, obstetric or perinatal variables based on the treatment received, the duration of T1D or maternal body mass index.
Characteristics and obstetric and perinatal outcomes in the 2 groups under study.
| CSII | MDI | P | |
|---|---|---|---|
| Mean ± standard deviation | |||
| Maternal age (years) | 31.23 ± 4.31 | 30.31 ± 5.3 | NS |
| Maternal BMI (kg/m2) | 24 ± 2.31 | 23 ± 1.9 | NS |
| Maternal weight gain (kg) | 9.6 ± 2.3 | 9.4 ± 2.8 | NS |
| Duration of DM (years) | 16.65 ± 8.36 | 13.73 ± 8.24 | NS |
| HbA1c, baseline (%) | 7.0 ± 1.3 | 7.5 ± 1.3 | NS |
| HbA1c, 1st trimester (%) | 6.6 ± 0.9 | 7.2 ± 1.2 | NS |
| HbA1c, 2nd trimester (%) | 6.2 ± 0.4 | 6.5 ± 0.6 | NS |
| HbA1c, 3rd trimester (%) | 6.2 ± 0.7 | 6.4 ± 0.7 | NS |
| Duration of pregnancy (weeks) | 36.71 ± 1.61 | 36.55 ± 3.34 | NS |
| Percentage | |||
| Macrosomia | 44% | 46.2% | NS |
| Retinopathy | 33.3% | 27.3% | NS |
| Caesarean delivery | 64.7% | 59.1% | NS |
| Polyhydramnios | 5.9% | 0% | NS |
| Preeclampsia | 23.2% | 9.5% | < .05 |
| Preterm birth | 27.7% | 31.8% | NS |
| IUGR | 23.5% | 0% | < .05 |
| Neonatal hypoglycaemia | 35.7% | 50.0% | NS |
| Neonatal hypocalcaemia | 7.1% | 5.5% | NS |
| Neonatal jaundice | 21.4% | 44.4% | NS |
| Neonatal respiratory distress | 35.7% | 33.3% | NS |
| Admission to neonatal unit at birth | 68.8% | 61.1% | NS |
BMI, body mass index; CSII, continuous subcutaneous insulin infusion; DM, diabetes mellitus; HbA1c, glycated haemoglobin; IUGR, intrauterine growth restriction; MDI: multiple dose injection; NS, not significant.
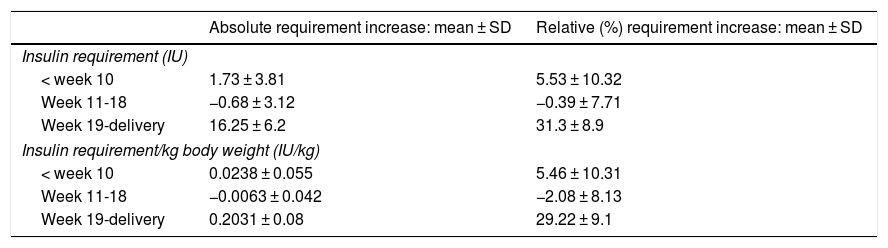
When we compared the immediate pregestational period with the first trimester of gestation in patients treated with CSII, we observed a significant increase in the daily capillary blood glucose levels measured in the first trimester (4.1 ± 1.1 vs 7.1 ± 1.3; P < .05) and the number of correction boluses administered per day (3.5 ± 1.1 vs 5.2 ± 0.9; P < .05). We also found increases in insulin requirements (in absolute terms and per kg of body weight) from week 1 to week 10 of gestation, followed by decreases between weeks 11 and 18 and increases from week 18 to delivery (P < .001) (Table 2).
Insulin requirements during pregnancy.
| Absolute requirement increase: mean ± SD | Relative (%) requirement increase: mean ± SD | |
|---|---|---|
| Insulin requirement (IU) | ||
| < week 10 | 1.73 ± 3.81 | 5.53 ± 10.32 |
| Week 11-18 | −0.68 ± 3.12 | −0.39 ± 7.71 |
| Week 19-delivery | 16.25 ± 6.2 | 31.3 ± 8.9 |
| Insulin requirement/kg body weight (IU/kg) | ||
| < week 10 | 0.0238 ± 0.055 | 5.46 ± 10.31 |
| Week 11-18 | −0.0063 ± 0.042 | −2.08 ± 8.13 |
| Week 19-delivery | 0.2031 ± 0.08 | 29.22 ± 9.1 |
SD, standard deviation; wk, week.
In addition, pregnant women managed with CSII that had infants with macrosomia had higher insulin requirements (absolute and per kg of body weight) throughout pregnancy (P < .05). We did not find any differences in the mean glucose level (±SD) or the percentage of blood glucose measurements outside the target range programmed in the CSII pump. Lastly, mothers managed with CSII whose babies had neonatal hypoglycaemia had greater insulin requirements (absolute and per kg of body weight) throughout pregnancy (P < .05), and a higher mean baseline blood glucose level (±SD).
In women with T1D, pregnancy causes rapid changes in insulin requirements that make it difficult to maintain good glycaemic control.4 Treatment with CSII offers greater flexibility to adapt in response to these changes, and its use is recommended by some authors, especially in the first trimester of pregnancy.6 In this regard, we ought to highlight the high percentage of women managed with CSII in our study cohort (46.1%), which exceeded the percentages reported for the general population and in studies specifically focused on women with pregestational T1D.
Pregestational glycaemic control is essential to achieve the best possible maternal and foetal outcomes. In our cohort, with a mean pregestational glycated haemoglobin (HbA1c) concentration of 7.30%, and despite improvements during gestation and the absence of severe acute decompensations of T1D, we still found a high incidence of complications during pregnancy and in the perinatal period (especially macrosomia), although it was similar to those reported in previous studies3 and these outcomes need to be interpreted in the context of the limitations intrinsic to the small sample size and retrospective design of our study. On the other hand, we found that glycaemic control was similar in both treatment groups, although the duration of diabetes was greater in the group of patients managed with CSII. While pregnant women managed with CSII had better baseline HbA1c values, these differences diminished and eventually disappeared in subsequent trimesters. In addition, the incidence of intrauterine growth restriction and preeclampsia was greater in the CSII group. This difference may seem contradictory in the context of a treatment method that allows better glycaemic control, but it has been described in the past in association with a greater difficulty in maintaining glycaemic control, duration of diabetes and complications in patients treated with CSII.7
The observed changes in insulin requirements, with a succession of increases and decreases, have been described in the previous literature,4 but not in a group exclusively treated with CSII, which makes our findings more precise and reliable. Last of all, the greater insulin requirements observed in mothers treated with CSII of infants with macrosomia could be explained by greater difficulties maintaining glycaemic control and more frequent need of correction boluses.
In conclusion, despite the creation of specific management units and the use of CSII in pregestational T1D, the incidence of maternal and foetal complications remains high. New methods need to be developed to improve metabolic control in these patients.
Please cite this article as: Bahíllo-Curieses MP, Matías del Pozo V, Álvarez Colomo C, Díaz-Soto G. Tratamiento insulínico, requerimientos de insulina y resultados perinatales en una cohorte de embarazadas con diabetes tipo 1. An Pediatr (Barc). 2021;94:107–109.