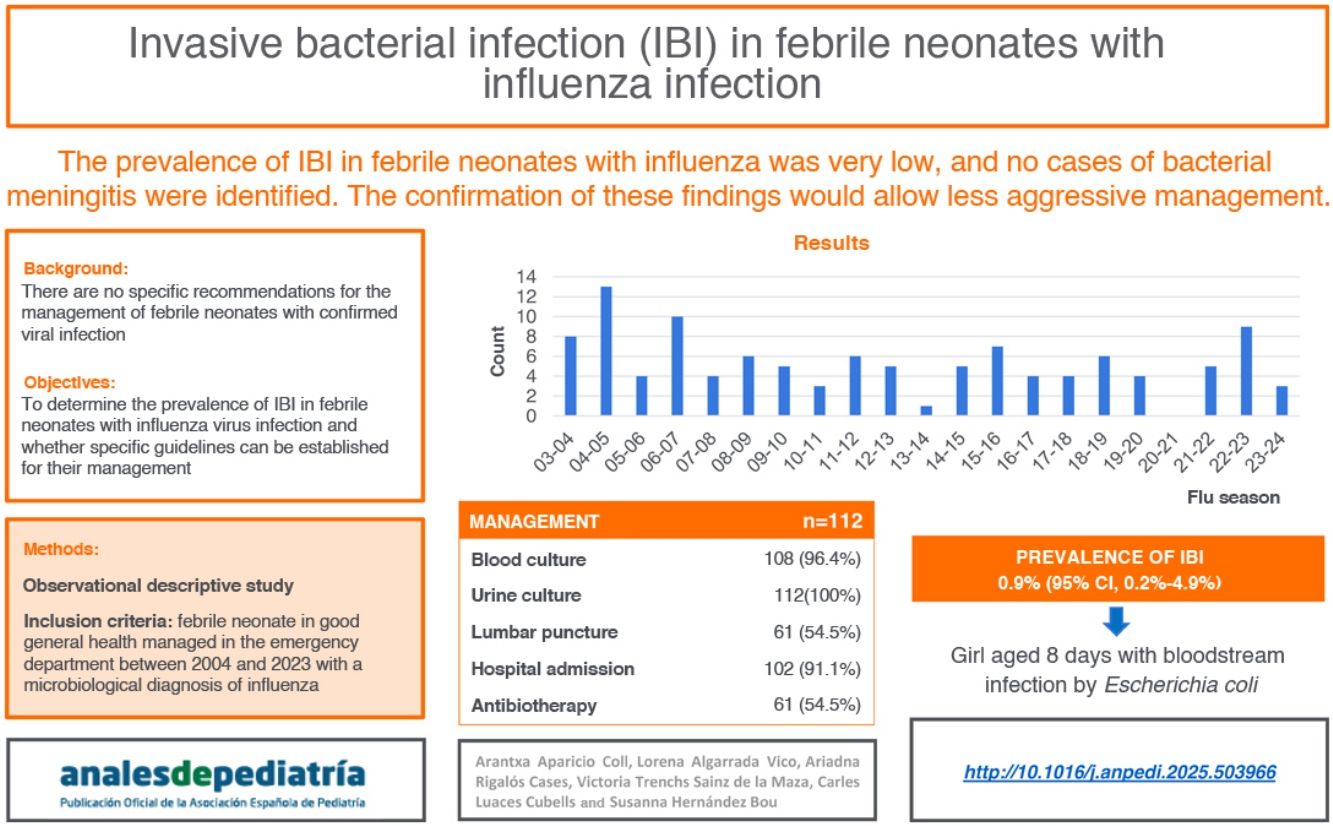
Febrile infants with a confirmed viral infection are at lower risk of an invasive bacterial infection (IBI). Several authors recommend individualizing screening in febrile infants aged 1 to 3 months, but there are currently no specific recommendations for neonates. The objective of our study was to determine the prevalence of IBI in febrile neonates with influenzavirus infection.
MethodsObservational descriptive study in febrile infants aged less than 29 days managed in the emergency department with a microbiological diagnosis of influenza over a 21-year period (2004–2023). We excluded patients with an abnormal pediatric assessment triangle or who had received antibiotherapy in the past week.
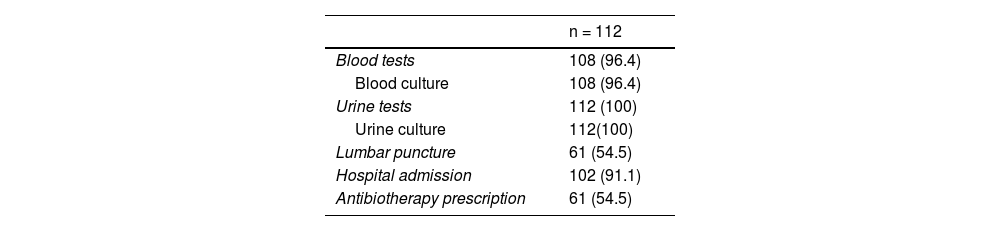
ResultsThe sample included 112 patients. Urine culture was performed in all, blood culture in 108 (96.4%) and lumbar puncture in 61 (54.5%). A total of 102 (91.1%) were admitted to hospital, of who 61 (59.8%) received antibiotics. An IBI was identified in one patient (prevalence 0.9%; 95% CI, 0.2%–4.9%): a female aged 8 days with a bloodstream infection by Escherichia coli. Additionally, 7 (6.3%, 95% CI: 3.1%–12.3%) patients presented with a urinary tract infection.
ConclusionsThe prevalence of IBI in febrile neonates with influenza infection is very low, with no detection of cases of bacterial meningitis. Confirmation of these results in a multicenter study, which could also identify associated risk factors, would allow a less aggressive approach of low-risk patients.
Los lactantes febriles con una infección vírica demostrada tienen menor riesgo de padecer una infección bacteriana invasiva (IBI). Diversos autores recomiendan individualizar su despistaje en los lactantes febriles de 1-3 meses de edad, no existiendo por el momento recomendaciones específicas para los neonatos. El objetivo del estudio es conocer la prevalencia de IBI en los neonatos febriles con infección por virus influenza.
Materiales y métodosEstudio descriptivo-observacional. Se incluyeron los pacientes febriles < 29 días atendidos en Urgencias durante 21 años (2004-2023) con diagnóstico microbiológico de gripe. Se excluyeron los pacientes con un triángulo de evaluación pediátrico alterado y aquellos que habían recibido antibiótico durante la semana previa.
ResultadosSe incluyeron 112 pacientes. Se realizó urocultivo a todos, hemocultivo a 108 (96,4%) y punción lumbar a 61 (54,5%). Ingresaron 102 (91,1%) casos, 61 (59,8%) de ellos con antibiótico. Se identificó un caso de IBI (prevalencia: 0,9%; IC 95%: 0,2-4,9%); niña de 8 días de vida con bacteriemia por Escherichia coli. Además, 7 (6,3%, IC 95%: 3,1-12,3%) pacientes presentaron una infección del tracto urinario.
ConclusionesLa prevalencia de IBI en los neonatos febriles con gripe es muy baja, no identificándose ningún caso de meningitis bacteriana. La confirmación de estos resultados en un estudio multicéntrico, que además permitiera identificar factores de riesgo, posibilitaría un manejo menos agresivo del subgrupo de pacientes con bajo riesgo.
The prevalence of serious bacterial infection (SBI) in infants aged less than 3 months with fever without source (FWS) is estimated at 5% to 20%, and the most frequent type is urinary tract infection (UTI).1 In Spain, the prevalence of invasive bacterial infection (IBI) is of approximately 2% to 4%; Escherichia coli and Streptococcus agalactiae are the most frequently involved bacteria in this age group, and together account for approximately 80% of the cases.2,3
The previous literature shows a lower incidence of SBI in febrile infants with a confirmed viral infection compared to febrile infants without it.3–14 Several authors have advocated for the inclusion of rapid tests to screen for viral infections in febrile infant management algorithms on account of their high diagnostic yield, recommending individualization of IBI screening in infants aged 1 to 3 months with a demonstrated viral infection.3,7,13,14 However, there is a dearth of specific recommendations for neonates . Thus, in our hospital, during the influenza season, the approach is to perform rapid testing for influenza in febrile infants aged 1 to 3 months and screening for UTI. If the rapid test is positive, screening for IBI is individualized, as is the use of empirical antibiotherapy and hospital admission (Appendix B).15 When it comes to febrile neonates with a positive rapid test for influenza, specific management recommendations have yet to be established, and more evidence is required in this age group for this purpose.
The aim of our study was to determine the prevalence of IBI in infants aged less than 29 days with FWS and influenza virus infection managed at a tertiary care pediatric hospital.
Material and methodsStudy designWe conducted an observational descriptive study in the emergency department (ED) of a tertiary care pediatric hospital that manages approximately 110 000 visits a year.
Sample selectionWe included patients aged less than 29 days managed at the ED for FWS during the influenza season (November-March) in the past 21 years (November 2003–March 2024) with a microbiological diagnosis of influenza infection (positive antigen or PCR test for influenza A or B in a nasopharyngeal aspirate sample). We excluded patients with an abnormal pediatric assessment triangle (PAT) in the ED because of their specific management,15 and those who had received antibiotherapy in the previous week.
As regards microbiological diagnosis, antigen tests were used from the beginning of the study period until the 2011–2012 influenza season, and molecular biology techniques thereafter.
According to our protocol for the management of febrile infants, patients < 29 days undergo a complete sepsis evaluation and are hospitalized with parenteral antibiotics. While awaiting the results of culture. Furthermore, during the influenza season, the protocol include performance of a rapid test for influenza, although there are no specific guidelines for management in the case of a positive result.15
Study variablesWe collected data on the following variables using a previously designed questionnaire,: age and sex, history of prematurity, perinatal factors associated with a high risk of infection (HRI) during pregnancy, underlying disease (genitourinary anomalies, history of severe systemic disease (complex heart disease, chronic lung disease, metabolic or neurologic diseases)), characteristics of fever (duration, maximum temperature, and presence of fever in the ED), other associated symptoms, additional tests performed (urine, blood, and/or cerebrospinal fluid [CSF] tests) and their results (laboratory and culture), indication for hospital admission, empirical antibiotherapy and course of disease (length of stay, other diagnoses, admission to intensive care unit, death, and return visit to ED in patients managed as outpatients).
For the purpose of the study, we considering the following perinatal factors as indicative of HRI: maternal vaginal/rectal culture positive for Streptococcus agalactiae, prolonged rupture of membranes (> 18 h), maternal chorioamnionitis, maternal UTI in the third trimester that was either not treated or whose treatment was not completed and/or maternal intrapartum fever.
Definitions- -
FWS: rectal temperature ≥ 38 °C or axillary temperature ≥ 37.5 °C documented at home or in the ED, where, after a detailed medical history and physical examination, the cause cannot be identified.
- -
IBI: isolation of a pathogenic bacterium in blood and/or CSF culture.
- -
SBI: growth of pathogenic bacteria in urine, blood, and/or CSF. Urinary tract infection was defined as growth with a count greater than 50 000 colony forming units per mL (CFU/mL) of a single pathogen isolated from urine collected by catheterization or growth with a count greater than 10 000 CFU/mL of a single pathogen from urine collected by catheterization combined with positive urinalysis results (visualization of bacteria in the gram stain by a microbiologist with nitrite-positive urine or leukocyturia).
Data analysis was conducted using IBM SPSS Statistics for Windows, version 29 (IBM Corp., Armonk, NY). Descriptive statistics were reported in terms of absolute frequencies or rates for categorical variables and in terms of median values with interquartile range (IQR) for continuous variables. The Kolmogorov-Smirnov test was used for the data distribution study. Statistical comparisons were made using Pearson χ2 test or Fisher exact test for categorical variables. The confidence interval (CI) was calculated at 95%. P values less than 0.05 were considered significant.
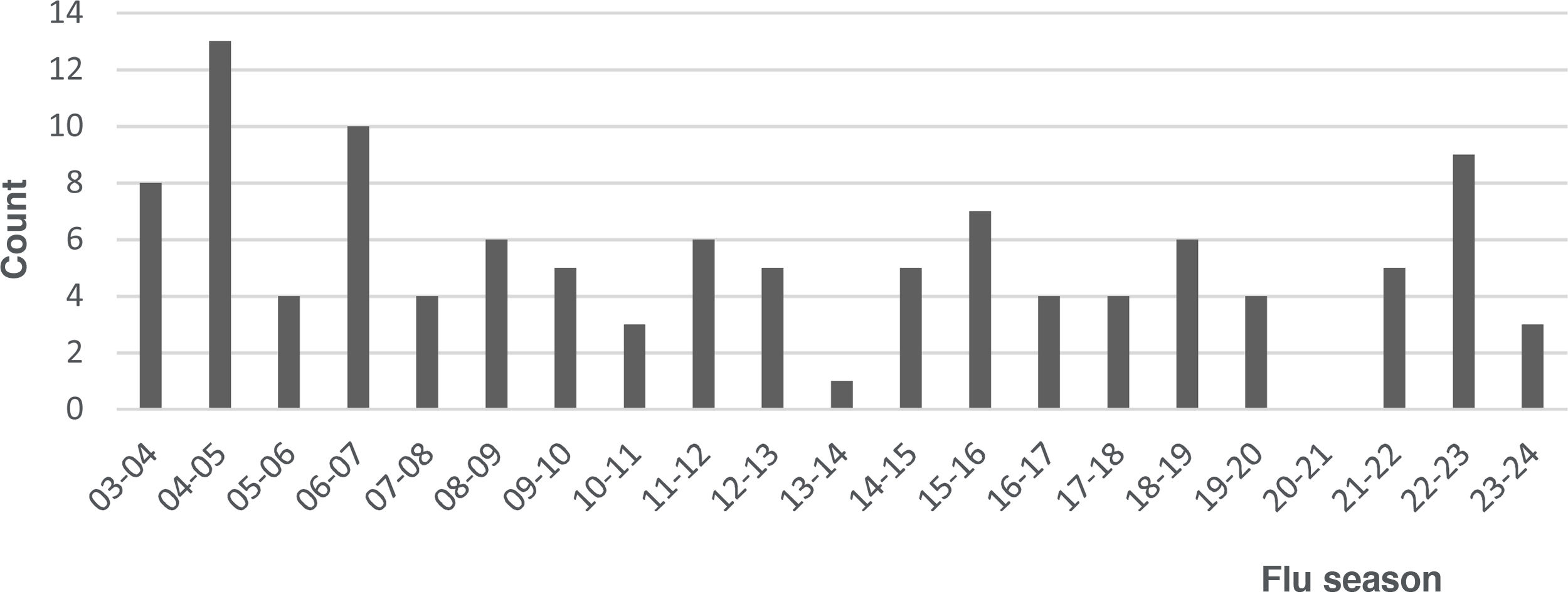
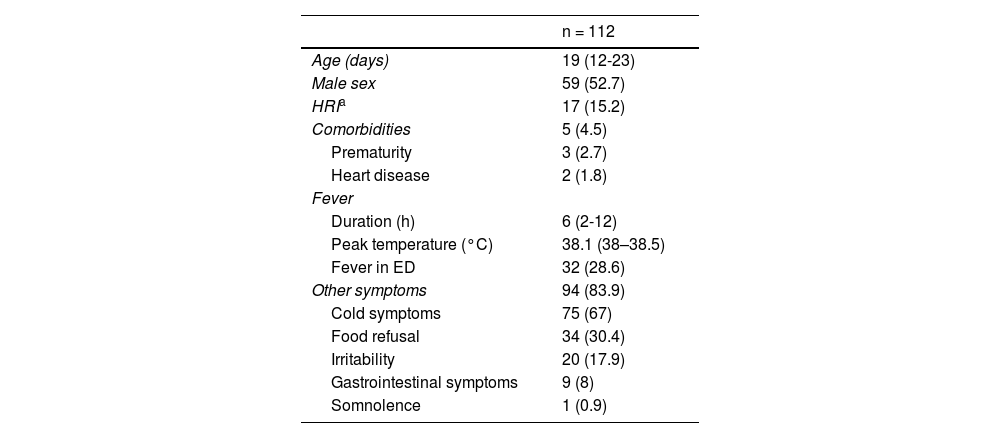
ResultsDuring the study period, 117 febrile neonates received a microbiological diagnosis of flu at the ED. Of this total, 5 (4.3%) met at least one exclusion criterion, resulting in a final sample of 112 patients. In 53 cases, the diagnosis was made by antigen tests and in 59 by molecular tests. Fig. 1 shows the distributions of cases over the study period; no cases were diagnosed during the 2020–2021 season, concurrent with the SARS-CoV-2 pandemic. Influenza A was identified in 94 cases (83.9%) cases and influenza B in 18 cases (16.1%). Table 1 summarizes the demographic and clinical characteristics of the sample.
Demographic and Clinical Characteristics of the Sample.
| n = 112 | |
|---|---|
| Age (days) | 19 (12-23) |
| Male sex | 59 (52.7) |
| HRIa | 17 (15.2) |
| Comorbidities | 5 (4.5) |
| Prematurity | 3 (2.7) |
| Heart disease | 2 (1.8) |
| Fever | |
| Duration (h) | 6 (2-12) |
| Peak temperature (°C) | 38.1 (38–38.5) |
| Fever in ED | 32 (28.6) |
| Other symptoms | 94 (83.9) |
| Cold symptoms | 75 (67) |
| Food refusal | 34 (30.4) |
| Irritability | 20 (17.9) |
| Gastrointestinal symptoms | 9 (8) |
| Somnolence | 1 (0.9) |
Abbreviations: ED, emergency department; HRI, high risk of infection.
Quantitative variables expressed as median (IQR) and categorical variables as n (%).
Some form of bacterial culture was performed in all patients, most frequently a urine culture (n = 112;100%). In 57 patients (50.9%), a PCR test for detection of enterovirus was performed in blood and/or CSF samples. Table 2 summarizes the management of the patients in the ED. By age group, 71.0% of neonates aged less than 14 days underwent lumbar puncture (LP) compared to 48.1% of those aged 14 to 28 days (P = .03), and there were no significant differences in the performance of LP between neonates aged less than 21 day versus neonates aged 21 to 28 days (55.9 vs 52.3%; P = .708). We also found no differences in the frequency of LP in relation to the history of HRI (76.5% vs 50.5%; P = .064), the duration of fever (< 6 h, 61.5% vs ≥ 6 h, 48.3%; P = .163) or the presence of associated symptoms (fever and no other symptoms, 61.1% vs fever and associated symptoms, 53.2%; P = .537).
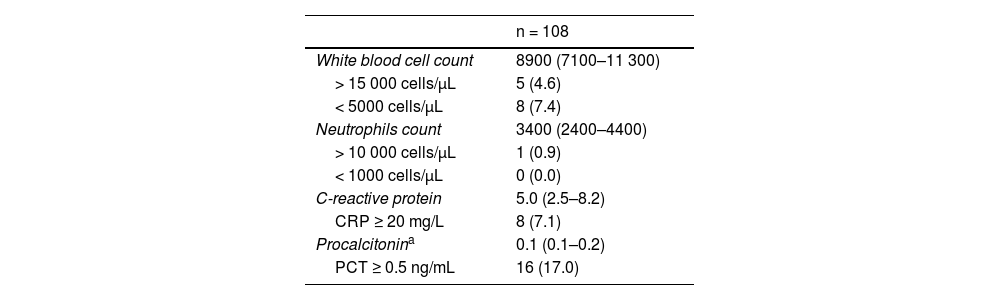
Table 3 presents the main laboratory findings. Eight patients (7.1%) had a C-reactive protein level (CRP) of 20 mg/L or greater (normal range, 20.1–81.4 mg/L). Out of 16 patients (17.0%) with a procalcitonin (PCT) level of 0.5 ng/mL or greater (normal range, 0.5–1 ng/mL), 13 (81.2%) had levels of 0.5 ng/mL.
Laboratory Findings in Emergency Department.
| n = 108 | |
|---|---|
| White blood cell count | 8900 (7100–11 300) |
| > 15 000 cells/μL | 5 (4.6) |
| < 5000 cells/μL | 8 (7.4) |
| Neutrophils count | 3400 (2400–4400) |
| > 10 000 cells/μL | 1 (0.9) |
| < 1000 cells/μL | 0 (0.0) |
| C-reactive protein | 5.0 (2.5–8.2) |
| CRP ≥ 20 mg/L | 8 (7.1) |
| Procalcitonina | 0.1 (0.1–0.2) |
| PCT ≥ 0.5 ng/mL | 16 (17.0) |
Quantitative variables expressed as median (IQR) and categorical variables as n (%).
A total of 102 patients (91.1%) were hospitalized, 61 (59.8%) of whom were admitted with empirical antibiotherapy (Table 2). Of the 8 patients with a CRP level of 20 mg/L or greater, 6 (75%) underwent LP and received antibiotherapy; the remaining 2 patients had CRP levels of 20.1 and 35.9 mg/L, respectively. Out of the 16 patients with PCT levels of 0.5 ng/mL or greater, 9 (56.3%) underwent LP and received antibiotherapy; the remaining 7 patients had PCT levels of 0.5 ng/mL. All neonates with a SBI received antibiotherapy on admission.
One case of IBI was identified (prevalence, 0.9%; 95% CI, 0.2%–4.9%). It corresponded to a female neonate with no perinatal factors associated with a HRI that presented with fever of 2 hours’ duration at 8 days post birth associated with mild cold symptoms who underwent a comprehensive sepsis evaluation at the ED, which yielded normal laboratory values, and was admitted to hospital with empirical antibiotherapy. A bloodstream infection by Escherichia coli was identified, while the urine and CSF cultures turned out negative. There were no cases of bacterial meningitis. Seven patients (6.3%, 95% CI, 3.1%–12.3%) had a UTI (involving E. coli in 4, Enterococcus faecalis in 2 and Klebsiella pneumoniae in 1), for an overall prevalence of PSBI of 7.1% (95% CI, 3.7%–13.5%). We did not find statistically significant differences in the prevalence of UTI between age groups: 9.7% in neonates under 14 days vs 4.9% in neonates aged 14 to 28 days (P = .393) and 7.4% in neonates under 21 days vs 4.5% in neonates aged 21 to 28 days (P = .702). Viral coinfection by enterovirus was also detected in three cases (2.7%).
During the hospital stay, three patients received a diagnosis of acute otitis media and one patient of bronchiolitis. All had favorable outcomes, and none of them required admission to the intensive care unit. The median length of stay was 72 hours (IQR, 48–96 h).
Of the 10 patients discharged home from the ED (77.3%), one returned to the ED within 24 hours due to persistent fever and was managed at the outpatient level without performance of additional tests; the urine and blood cultures from the initial visit in this patient were both negative.
DiscussionIn our study, which evaluated the co-occurrence of IBI in a sample of 112 febrile neonates in good general health and with infection by influenza, we only identified one patient (0.9%) with a bloodstream infection.
In relation to the distribution of cases throughout the study period, we ought to highlight the absence of cases during the first year of the SARS-CoV-2 pandemic, with a subsequent reemergence in the post-pandemic period. Several authors have described the low circulation of influenza and other respiratory viruses during the first year of the pandemic, chiefly attributing it to the public health measures implemented to contain the transmission of SARS-CoV-2.16,17
The identification of influenza virus motivated a less aggressive approach to the management of these patients in reference to the guidelines for the evaluation of febrile neonates in our hospital.15 It is worth noting that rapid tests were performed during the influenza season, when their positive predictive value is higher due to the high prevalence of the infection. When it came to screening of SBI, urine and blood cultures were performed in nearly all patients and CSF culture in a little more than half. The latter finding has to be interpreted with caution, as we counted only the patients in whom CSF culture was performed and did not include cases in which LP was performed but CSF was not obtained or could not be analyzed due to the presence of blood in the sample. We expect that, given the difficulty of the LP procedure in these very young patients, the actual proportion of LP was higher than reported.18 Similarly, while most patients were admitted to hospital, antibiotherapy was prescribed to approximately 60%.
Performance of LP was significantly more frequent in neonates under 14 days compared to older infants, but we found no differences between other age subsets or based on any other of the clinical variables under study, although we found a tendency toward more frequent performance of LP in neonates with a history indicative of HRI (3 out of 4). The fact that nearly all patients were hospitalized, which allowed close clinical monitoring, could largely explain the lower-than-expected frequency of LP in patients with a short duration of fever, despite the greater difficulty in interpreting laboratory results in these cases. We also ought to mention that there were some patients with biomarkers in the abnormal range, especially PCT, who did not undergo LP and in whom antibiotherapy was not initiated. In the previous literature, several authors have underscored the variability in the adherence to clinical guidelines for the management of fever in young infants.19–21 In our case, the fact that the biomarker elevation was mild, combined with the confirmation of a viral infection, probably played a role in the lack of adherence to the protocol.
The lack of specific guidelines for the management of febrile neonates with confirmed viral infection was highlighted in the study published by Simone et al.,22 which estimated the probability of performance of LP in febrile neonates with bronchiolitis and found substantial variability between emergency care physicians in Canada and in United Kingdom and Ireland. In line with our results, in recent years some authors have questioned the routine screening of meningitis in febrile neonates with bronchiolitis. Thus, De Rose et al.,23 in a study that included 242 patients aged less than 3 months hospitalized for bronchiolitis, of who 158 were neonates, proposed awaiting the results of respiratory panels before deciding on the performance of LP, as no cases of bacterial meningitis were diagnosed in their case series. Similarly, Berk et al.,24 in a review of the subject within the “Things We Do for No Reason” series, concluded that in febrile infants with bronchiolitis aged less than 3 months considered to be at low risk of IBI based on febrile infant risk-stratification algorithms, evaluation of bacterial meningitis with a LP would be unnecessary given that, in such cases, the risk of harm associated with LP (trauma, hospitalization, antibiotherapy, costs, family stress)18,25,26 probably outweighs the potential benefits of the intervention.
Several algorithms for the evaluation of febrile neonates propose individualization of LP and initiation of empirical antibiotherapy in the group aged 21 to 28 days.27–29 In our sample, due to the low prevalence of IBI, we were unable to analyze age subsets as a risk factor.
A limited number of patients had clinically significant elevation of acute phase reactants. The patient with a bloodstream infection, who was brought to the ED with a fever of two hours' duration, had no any laboratory abnormalities suggestive of IBI. This finding highlights the need for caution in the evaluation of these patients, given how quickly they tend to be brought to the ED upon onset of fever, a fact that could affect the performance of clinical prediction rules, especially in cases with a shorter duration of fever.30,31
Our study had limitations, among them those intrinsic to its retrospective design: since it was based on electronic health records, some clinical data may have been lost. In addition, it was conducted in a single tertiary care children’s hospital, so the described approach to management may not be representative of customary clinical practice in other care settings. Third of all, we analyzed management in reference to the guidelines for the assessment of febrile neonates in our hospital; however, having a control group of febrile neonates with a negative rapid test would have reflected everyday clinical practice more faithfully. Furthermore, the exclusion of patients with an abnormal PAT on account of their specific management could have led to the underestimation of the prevalence of IBI in this population. Similarly, the lack of universal screening for IBI, especially the fact that LP was not performed in all patients, may have also led to underestimation of the prevalence; however, this is unlikely on account of the favorable outcome of patients who did not undergo LP. Last of all, the low prevalence of IBI detected in the sample did not allow for the analysis of risk factors that would allow identification of subsets of patients at increased risk.
In conclusion, the prevalence of IBI in febrile neonates with confirmed influenza is very low, and no cases of bacterial meningitis were identified in our sample. Confirmation of these results in a multicenter study, in addition to allowing the identification of risk factors, would make less aggressive management possible in a subset of patients, replacing routine LP and empirical antibiotic therapy with close clinical monitoring.
CRediT authorship contribution statementThe authors conducted the research and therefore confirm their authorship. They participated in its conception and design, the analysis and interpretation of the data and the drafting and editing of the manuscript, and all approved the final text that was submitted to Anales de Pediatría.
Ethical considerationsThe study was approved by the Research Ethics Committee of the Fundació Sant Joan de Déu (PIC-204-21). Due to the characteristics of the study, the Committee concluded that it met the criteria for a waiver of informed consent.
FundingThis research did not receive any external funding.
The authors have no conflicts of interest to declare.
Previous meeting: Preliminary results of this study were presented as an oral communication at the XXVI Annual Meeting of the Sociedad Española de Urgencias de Pediatría; June 16–18, 2022; Pamplona, Spain.