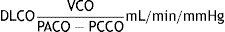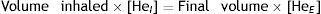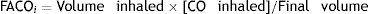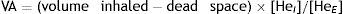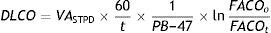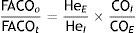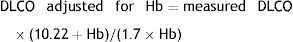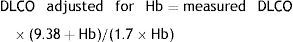The diffusion capacity is the technique that measures the ability of the respiratory system for gas exchange, thus allowing a diagnosis of the malfunction of the alveolar-capillary unit. The most important parameter to assess is the CO diffusion capacity (DLCO). New methods are currently being used to measure the diffusion using nitric oxide (NO). There are other methods for measuring diffusion, although in this article the single breath technique is mainly referred to, as it is the most widely used and best standardised.
Its complexity, its reference equations, differences in equipment, inter-patient variability and conditions in which the DLCO is performed, lead to a wide inter-laboratory variability, although its standardisation makes this a more reliable and reproductive method.
The practical aspects of the technique are analysed, by specifying the recommendations to carry out a suitable procedure, the calibration routine, calculations and adjustments. Clinical applications are also discussed. An increase in the transfer of CO occurs in diseases in which there is an increased volume of blood in the pulmonary capillaries, such as in polycythemia and pulmonary haemorrhage. There is a decrease in DLCO in patients with alveolar volume reduction or diffusion defects, either by altered alveolar-capillary membrane (interstitial diseases) or decreased volume of blood in the pulmonary capillaries (pulmonary embolism or primary pulmonary hypertension). Other causes of decreased or increased DLCO are also highlighted.
La capacidad de difusión es la técnica que mide la capacidad del aparato respiratorio para realizar el intercambio gaseoso y así diagnosticar la disfunción de la unidad alvéolo-capilar.
El parámetro más importante a evaluar es la capacidad de difusión del CO (DLCO). Actualmente hay nuevos métodos para medir la capacidad de difusión utilizando óxido nítrico (NO). Existen diferentes métodos de medida, aunque en este artículo nos referiremos sobre todo a la técnica de la respiración única, la más utilizada y mejor estandarizada.
Su complejidad, sus ecuaciones de referencia, las diferencias en equipamiento, la variabilidad interpacientes y las condiciones en las que se realiza hacen que exista una gran variabilidad interlaboratorio, habiéndose realizado estandarizaciones para hacer este método más fiable y reproducible.
Se analizan los aspectos prácticos de la técnica, especificando las recomendaciones para la realización de un procedimiento adecuado, sistemática de calibración y cálculos y ajustes necesarios. También se exponen sus aplicaciones clínicas.
Se produce un aumento de la transferencia de CO en las enfermedades en las que existe un aumento del volumen sanguíneo en los capilares pulmonares, en la policitemia y en la hemorragia pulmonar. Existe una disminución de la DLCO en los pacientes con reducción del volumen alveolar o en los defectos de difusión, ya sea por alteración de la membrana alvéolo-capilar (enfermedad intersticial) o por disminución del volumen de sangre en los capilares pulmonares (embolia pulmonar o hipertensión pulmonar primaria). También se exponen otras causas de disminución o aumento de la DLCO.
The main function of the lungs is to establish the exchange of oxygen (O2) and carbon dioxide (CO2) between tissue and ambient air. Gas exchange depends on three processes: ventilation, diffusion and pulmonary perfusion.
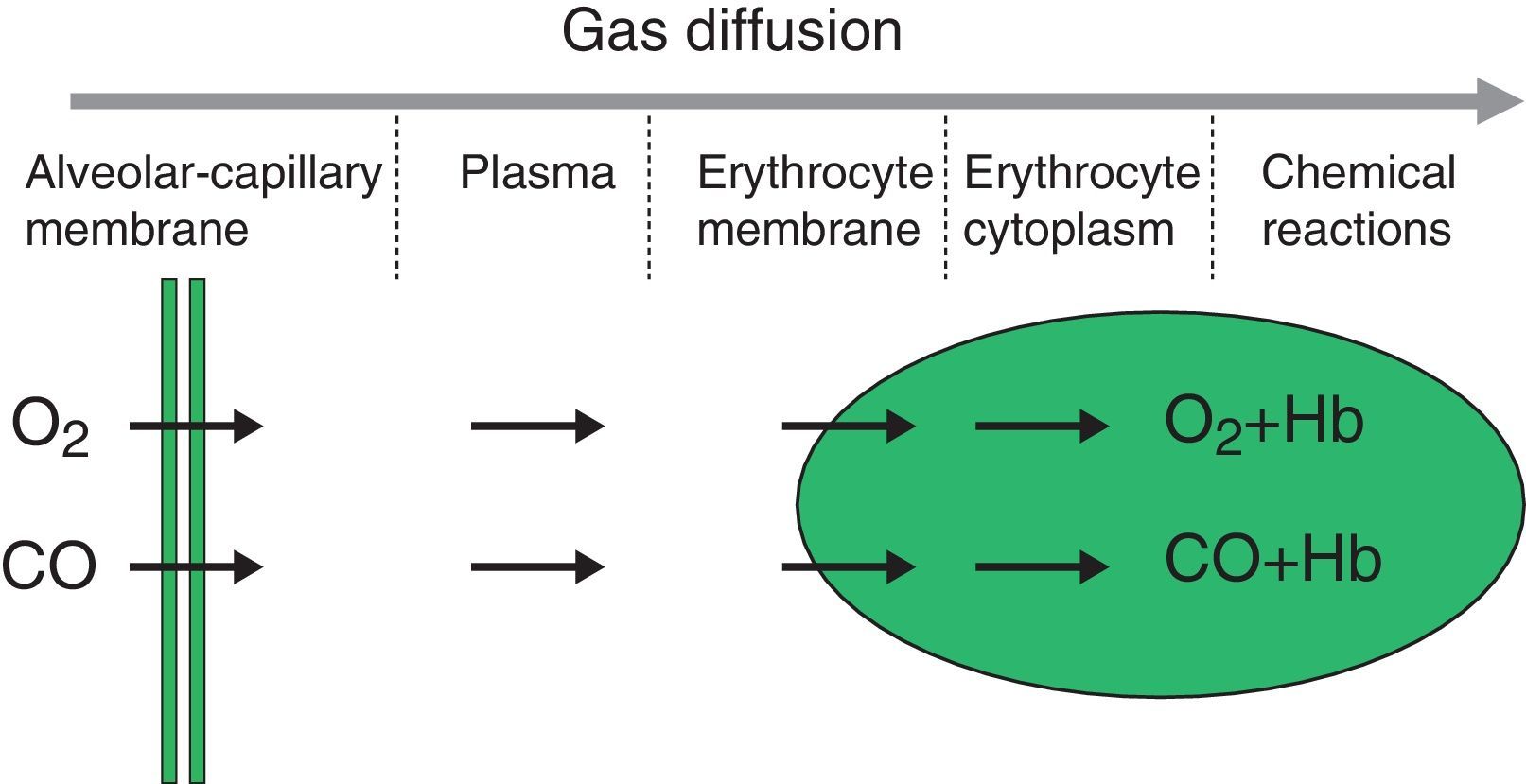
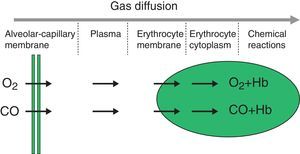
The diffusion process is defined as the flow of particles from an area of greater concentration to an area of lesser concentration. The rate of transfer by diffusion of any gas through a membrane (Fig. 1) is directly proportional to the surface area of the membrane and inversely proportional to its thickness; it also depends on the molecular weight, pressure gradient and solubility of the gas, the ventilation-perfusion ratio of the lung units, the rate of combination with haemoglobin and the haemoglobin values. The diffusion rate of CO2 is 20 times higher than that of O2.
Measuring diffusion provides information on gas transfer between the alveoli and the blood of the pulmonary capillaries and we generally refer to it as diffusion capacity (DLCO).1,2 To assess the functional integrity of the diffusion process a gas must be used that is not present in venous blood, that has an affinity for haemoglobin and that is soluble in blood. The gas universally used is carbon monoxide (CO).
CO diffusing capacity (DLCO) is the uptake of this gas per minute (VCO: mL of CO transferred per minute) in relation to the CO gradient through alveolar-capillary membrane (the difference between the partial pressures of CO in the alveoli [PACO] and in the capillary blood [PCCO] in mmHg).
There are currently new methods for measuring diffusion capacity using nitric oxide (NO), as this gas is independent of O2 pressure and haematocrit and has a greater affinity for haemoglobin (Hb) than CO. Moreover, DLNO is less influenced by capillary blood volume than DLCO and represents the true diffusing capacity of the alveolar-capillary membrane. In addition, the DLNO/DLCO ratio could be a good indicator of impairment in gas exchange, which would be an improvement on the tests currently used in clinical practice.3,4 In cystic fibrosis, combined analysis with CO and NO could improve its functional assessment.5 Research is also being conducted on the influence of breath-hold time on diffusion capacity of NO compared with CO.6 The use of NO is therefore a promising method.7
Techniques for measuring diffusionThere are three main methods for measuring DLCO: (a) the rebreathing technique, (b) the multiple-breath or steady state technique, and (c) the single-breath (SB) technique. The most widely used and best standardised method is the last of these.8
- (a)
Rebreathing method: the patient breathes for 10–15s into a small bag containing CO and helium (He). Little used in clinical practice.
- (b)
Multiple-breath technique: many children have difficulties in holding their breath for 10s or have a vital capacity (VC) lower than 1.5L, so that they cannot perform the single-breath technique; other techniques have therefore been devised.9
The most commonly used is called the multiple-breath or steady state technique, in which the patient is told to take normal breaths at tidal volume after being connected to a closed system filled with a gas mixture containing 5% He and 0.3% CO, so that the disappearance of CO from the system and the fall in He due to dilution are continuously monitored. During the procedure CO2 is absorbed, while O2 is kept between 20% and 22%. The ventilation of the child is measured with a displacement transducer connected to the bellows or piston, which moves as the patient breathes while the He, CO and O2 concentrations are continuously monitored. As the patient is connected to the system at functional residual capacity (FRC) level, this FRC is determined by He dilution, whereas diffusing capacity is calculated from the exponential decay in CO. The results depend on alveolar ventilation, and the breathing pattern must be as stable as possible.10
An inhalation bag with a two-way valve, containing a mixture of CO at a certain concentration, can also be used. The patient breathes several times and the exhaled gas is collected and analysed. The CO uptake is obtained by multiplying the difference between the inhaled and exhaled concentrations by the ventilation volume per minute. The partial alveolar pressure of CO at which the transfer is made also needs to be measured.11
There are predicted values for DLCO and DLCO/VA with this technique for patients aged from 6 to 18.12
It is to be expected that the use of new systems, which analyse CO breath by breath, not estimating its concentration but determining it in real time, may avoid errors that arise with the other techniques.
Nevertheless, new validation and standardisation studies of this technique are needed.
- (c)
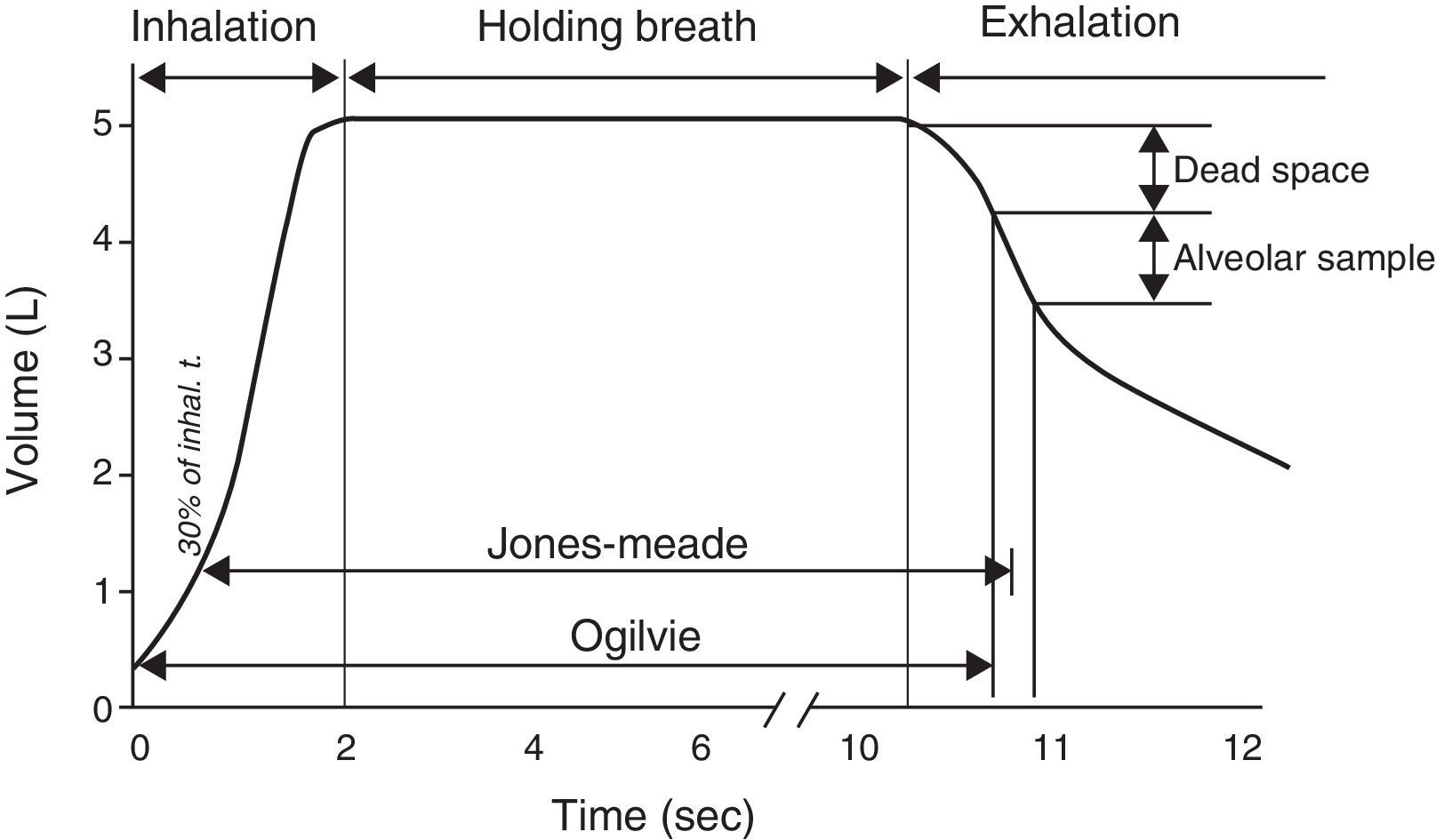
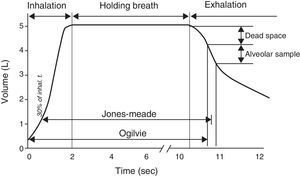
Single-breath method (DLCOSB): measuring DLCO per single breath involves making the patient exhale to residual volume (RV) and then take in a rapid breath of a gas containing CO 0.3%, He 10%, O2 21% and nitrogen in equilibrium, to over 90% of vital capacity. After rapid inhalation of the gas, the breath is held for 10s at total lung capacity (TLC) to allow it to diffuse and then the patient is made to exhale rapidly. The first part (between 0.5 and 1L) is discarded, as it corresponds to the dead space that has not undergone the diffusion process, and the second, representing the gas that has been in the alveoli (alveolar fraction), is used. In this second fraction the final concentration of CO and He is determined. The initial concentration of CO in the alveolar gas is not the same as the concentration of CO in the inhaled gas administered to the patient, since this gas has to be diluted in the air present in the lung after maximum exhalation (RV) and air in the dead space in the system. Since only the concentration of the gas administered is known and not that present in the alveolar space, a tracer gas (generally He) is used to calculate the latter. He is an inert gas, which is not diffused through the alveolar-capillary membrane. Therefore, if we know the initial concentration of He in the gas administered (HeI) and the volume of gas inhaled (measured with the spirometer), the final concentration of helium (HeE), measured in the exhaled air, will depend on the final volume in which the He has been dissolved (alveolar volume+dead space).
The main criticism of this method is that it measures diffusion in a very unphysiological situation: during a maximum inhalation and while the breath is held, which does not occur in normal breathing at tidal volume.
Another of the problems with DLCO is that we are using a single value to express the diverse properties of millions of respiratory units, which differ according to the region of the lung.
Single-breath DLCO depends on the amount of lung tissue involved in the gas exchange. For this reason it is advisable in these patients to assess diffusing capacity per unit of lung volume, KCO (DLCO/VA).13,14
The complexity of the technique, the reference equations for the test, differences in equipment, interpatient variability and the conditions in which the test is performed mean that there is a high degree of interlaboratory variability,15 much greater than with other pulmonary function measurements, and standardisation is therefore essential.2,8,16,17 Reference values have been established in healthy white children aged from 5 to 19 for DLCO and VA.18
We must not forget that the absolute values obtained with these two techniques, single- and multiple-breath, are different, as they are performed at total lung capacity (TLC) and functional residual capacity (FRC) respectively, and diffusion capacity is related to the surface area assessed, which is lower in the latter; therefore when the two techniques are compared the results have to be calculated as z-scores or standard deviations from the corresponding predicted values.
Practical aspects of the techniquePreparation of the patientThe patient must refrain from smoking for 24h before the test, avoid alcohol for at least 4h and not take exercise. He or she will remain seated for at least 5min before the test and throughout the procedure.19
If the patient requires oxygen it is preferable to discontinue it at least 5min before beginning the test. If this is not possible, the results must be assessed with caution.
EquipmentThe patient breathes through a pneumotacograph, which measures the volume of air inhaled and is connected to a three-way valve, which initially allows the patient to breathe ambient air; subsequently, during forced inhalation, the way is opened to the gas cylinder, and during exhalation it is opened to the bag in which the alveolar gas sample is collected. During the 10s for which the breath is held there must be a shutter that prevents exhalation and also a pressure sensor to assess whether the patient is performing Valsalva manoeuvres (exhalation) or Müller's manoeuvres (inhalation), which modify pulmonary capillary blood volume. The Valsalva manoeuvre reduces DLCO and Müller's manoeuvre increases it.20 Finally, gas analysers are needed to ascertain the concentration of exhaled CO and He. The system must have a dead space of less than 100mL. All the apparatuses must be calibrated daily.21
Test procedure- 1)
Explain the manoeuvre. It is advisable to carry out a simulation first without gas inhalation.
- 2)
The mouthpiece is placed in the mouth and the clip on the nose and the patient is asked to breathe calmly.
- 3)
The patient is told to exhale to RV. If there is a major obstructive disease it is recommended that exhalation should be limited to 6s.
- 4)
A rapid breath is taken to TLC:
- a)
The inspiratory volume (IV) must be at least 90% of the largest previous VC (spirometry must be performed beforehand as a guide).
- b)
The inspiration must be rapid enough for 90% of the IV to be inhaled within 1.5–2s in healthy individuals and in less than 4s in patients with obstructive disease. If the inhalation lasts for 5s, DLCO increases by around 13%.
- a)
- 5)
The breath must be held for 10s.
- a)
During this time no expiratory or inspiratory effort must be made against the shutter.
- a)
- 6)
After this the patient must exhale rapidly.
- a)
The first part (about 750mL) is discarded. If the patient has a VC<2L this can be reduced to 500mL.
- b)
The volume analysed must be between 500 and 1000mL and must be exhaled in less than 4s.
- a)
- 7)
The patient is told to remove the nose clip and remain seated.
- 8)
The test must be repeated until at least two values are achieved that agree within 10% and below 3mL/min/mmHg. At least 4min must elapse between each test. In patients with major obstruction it may be necessary to wait up to 10min between tests for the gases to be completely eliminated. It may be useful to ask the patient to take deep breaths so as to eliminate the gas more effectively.
- 9)
Calculations:
Given that diffusing capacity is calculated per unit of time (mL of CO absorbed per minute), exact calculation of the time the patient holds his or her breath, which is the time during which CO diffusion takes place, is crucial.22,23 The Jones–Meade method for measuring the time starts counting from 30% of the inhalation time and stops at 50% of the alveolar gas sample collection time (Fig. 2).
In patients with obstruction it may be preferable to perform the test after using a bronchodilator, although if heart rate increases, volume per minute also increases, raising DLCO. For this reason it is advisable to carry out the test at least 30min after administering the bronchodilator.
AdjustmentsAdjustment for haemoglobin (Hb)Patients with anaemia have a reduced DLCO because CO uptake by erythrocytes is lower. The Cotes method24 of correction for Hb makes it possible to calculate adjusted DLCO in adult males with Hb greater than 14.6g/dL or in women and in children under 15 with Hb<13.4g/dL.
Males 15 years of age and older:
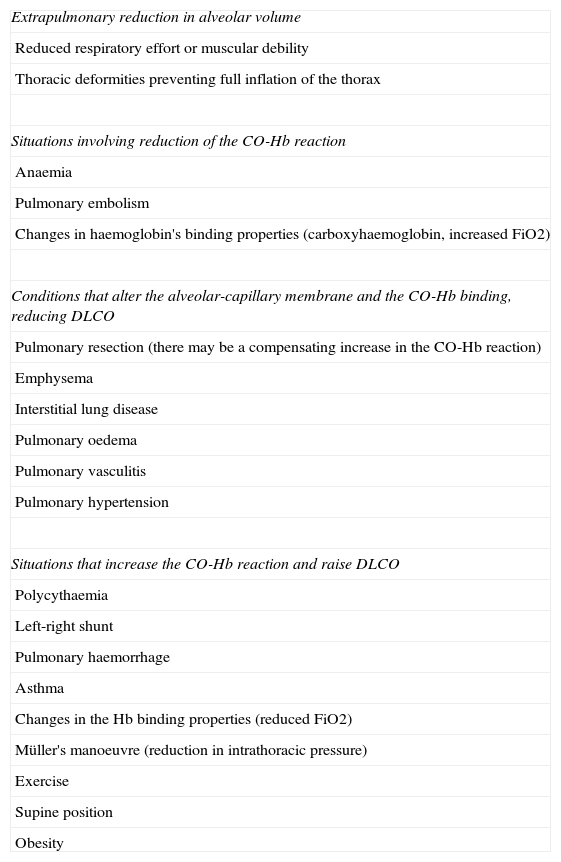
Children aged <15 years and women:It is advisable to put both values in the report: the measured DLCO and the DLCO adjusted for Hb.Clinical applicationsCO transfer can be either increased or reduced in various processes (Table 1).8
Processes associated with alterations in DLCO.
| Extrapulmonary reduction in alveolar volume |
| Reduced respiratory effort or muscular debility |
| Thoracic deformities preventing full inflation of the thorax |
| Situations involving reduction of the CO-Hb reaction |
| Anaemia |
| Pulmonary embolism |
| Changes in haemoglobin's binding properties (carboxyhaemoglobin, increased FiO2) |
| Conditions that alter the alveolar-capillary membrane and the CO-Hb binding, reducing DLCO |
| Pulmonary resection (there may be a compensating increase in the CO-Hb reaction) |
| Emphysema |
| Interstitial lung disease |
| Pulmonary oedema |
| Pulmonary vasculitis |
| Pulmonary hypertension |
| Situations that increase the CO-Hb reaction and raise DLCO |
| Polycythaemia |
| Left-right shunt |
| Pulmonary haemorrhage |
| Asthma |
| Changes in the Hb binding properties (reduced FiO2) |
| Müller's manoeuvre (reduction in intrathoracic pressure) |
| Exercise |
| Supine position |
| Obesity |
CO transfer is increased in situations in which there is an increase in blood volume in the pulmonary capillaries (exercise, left-right shunts), polycythaemia and pulmonary haemorrhage (falsely elevated DLCO). Occasionally there is a rise in DLCO in asthmatics, due to an increase in pulmonary blood volume though negative intrathoracic pressure produced after rapid inhalations.
DLCO is decreased in patients with a reduction in alveolar volume or in diffusion defects (alteration of the alveolar-capillary membrane [interstitial lung disease: idiopathic pulmonary fibrosis, extrinsic allergic alveolitis, scleroderma, sarcoidosis, asbestosis] or decreased pulmonary capillary blood volume [pulmonary embolism or primary pulmonary hypertension]). In pulmonary emphysema DLCO is characteristically reduced by loss of alveolar-capillary membrane surface area secondary to alveolar rupture, giving rise to an increase in TLC with a decreased KCO (DLCO/VA). In congestive heart failure the reduction in DLCO may be secondary to interstitial oedema. Other causes of decreased DLCO are anaemia, renal failure, smoking and marijuana use.
In patients with restrictive diseases, especially in children with deformities of the rib cage or neuromuscular diseases in which normal lungs are restrictive because of deformity or muscular weakness, the surface area for diffusion is relatively large per unit of pulmonary volume, and for this reason DLCO may be normal. The patient's DLCO/VA should therefore be compared with reference values based on his or her (restrictive) TLC rather than his or her theoretical TLC.25
In cystic fibrosis both raising and lowering of DLCO may be found. Initially the transfer factor may be elevated by an increase in the amount of blood reaching the lung, due to fluctuations in pleural pressure secondary to bronchial obstruction; later, with the onset of pulmonary heart disease, pulmonary microcirculation is impaired and diffusion is gradually reduced.26
Indications for the DLCO test are set out in Table 2.21
Indications for DLCO measurement.
| 1. Evaluation and followup of diseases affecting lung parenchyma (those associated with drug reactions, pneumoconiosis or sarcoidosis) |
| 2. Evaluation and followup of emphysema |
| 3. Differentiating between chronic bronchitis, emphysema and asthma |
| 4. Assessment of pulmonary involvement in systemic diseases |
| 5. Assessment of cardiovascular diseases |
| 6. Prediction of arterial desaturation during exercise in some patients with lung disease |
| 7. Assessment and quantification of the degree of disability associated with pulmonary fibrosis or with emphysema |
| 8. Assessment of the pulmonary effects of chemotherapy agents or other drugs that induce pulmonary dysfunction |
| 9. Assessment of pulmonary haemorrhage |
| 10. As an early indicator of certain pulmonary infections that cause diffuse pneumonitis (e.g., Pneumocystis pneumonia) |
In practice the main indications in paediatrics are as follows10:
Monitoring of treatments that are toxic to the lungsChemotherapy (methotrexate, nitrofurantoin, azathioprine, penicillamine, cyclophosphamide and especially bleomycin) can produce a major decrease in DLCO, which must be monitored.
Chest radiation can cause irreversible diffusion impairment.
Treatments for autoimmune or rheumatological diseases can also impair pulmonary diffusion.
Immunosuppressants used after organ transplants have been associated with obstructive and restrictive lung disease.
Diagnosis and monitoring of patients with chronic interstitial lung diseaseImpairment of diffusing capacity is one of the initial signs of interstitial lung disease and a fundamental indicator of clinical course and response to treatment.
Monitoring of children with diseases that cause pulmonary bleedingIn primary pulmonary haemosiderosis, Goodpasture syndrome and granulomatosis with polyangiitis, an increase in diffusing capacity can predict relapse or indicate progression in asymptomatic patients. Moreover, pulmonary haemosiderosis that does not respond to treatment may eventually give rise to pulmonary fibrosis, leading to decreased diffusion.
Please cite this article as: Posadas AS, Asensi JRV, de MirMessa I, Prado OS, Larramona H. Medición de la difusión de CO (II): estandarización y criterios de calidad. An Pediatr (Barc). 2015;83:137.e1–137.e7.