Prenatal corticosteroids reduce neonatal mortality and morbidity; however, there are few studies in developing countries, and with inconsistent results. The purpose of this study was to quantify the frequency of the use of prenatal corticosteroids and to estimate its effect on the morbidity and mortality of premature newborns.
MethodsA retrospective cohort study was performed on premature newborns selected from a census conducted between January 2016 and August 2017. The use of corticosteroids was taken from the maternal records, and the dependent variables from the neonatal records. An analysis was made of the relationship using logistic regression, adjusted to gestational age and weight.
ResultsThe study included 1083 premature infants of which 53.3% were male. The mean gestational age was 33.4 weeks. Corticosteroids were received by 42%, with latency ≥24h in 23.6% and ≥48h in 13.8%. Respiratory distress syndrome was observed in 35% (379/1083), early neonatal sepsis in 4.4% (48/1083), late neonatal sepsis in 10.7% (116/1083), intraventricular haemorrhage in 15.1% (137/908), chronic lung disease in 51.4% (165/321), and death in 22.3% (242/1083). Prenatal corticosteroids decreased the risk of death in children under 34 weeks (OR 0.63, 95% CI 0.40–0.98). The decrease was greater if they presented with latency ≥48h (OR 0.40, 95% CI 0.20–0.80). The rest of the dependent variables were not modified by the intervention.
ConclusionsIn preterm infants, 42% received antenatal corticosteroids. In those with less than 34 weeks, there was a decrease in the risk of death without changes in morbidity.
Los corticosteroides prenatales disminuyen la morbimortalidad neonatal, sin embargo, existen pocos estudios en países en vías de desarrollo, con resultados no consistentes. El objetivo fue cuantificar la frecuencia del uso de corticosteroides prenatales y estimar su efecto en la morbimortalidad de recién nacidos prematuros.
MétodosEstudio de cohorte retrospectivo; se seleccionaron los recién nacidos prematuros de un censo realizado entre enero de 2016 y agosto de 2017. De los expedientes maternos se registró el uso de corticosteroides; y de los expedientes de los neonatos se indagó las variables dependientes. La asociación se analizó con regresión logística, ajustada a la edad gestacional y el peso.
ResultadosSe estudiaron 1.083 prematuros, el 53,3% de género masculino; la edad gestacional promedio fue 33,4 semanas. Recibieron corticosteroides el 42%, con latencia ≥24horas el 23,6% y ≥48horas el 13,8%. Presentaron síndrome de dificultad respiratoria el 35% (379/1083), sepsis neonatal temprana el 4,4% (48/1083), sepsis neonatal tardía el 10,7% (116/1083), hemorragia intraventricular el 15,1% (137/908), enfermedad pulmonar crónica el 51,4% (165/321) y muerte el 22,3% (242/1083). Los corticosteroides prenatales disminuyeron el riesgo de muerte en menores de 34 semanas (OR: 0,63, IC 95%: 0,40-0,98); el decremento fue mayor si presentaron latencia ≥48horas (OR: 0,40; IC 95%: 0,20-0,80). El resto de variables dependientes no se modificó por la intervención.
ConclusionesEl 42% de los prematuros recibe corticosteroides prenatales. En menores de 34 semanas se observó una disminución del riesgo de muerte sin modificación en la morbilidad.
In recent decades, the risk of death during the neonatal period has decreased by 28% worldwide from 33.2 to 23.9 deaths per 1000 live births,1 although the number of deaths continues to be higher in low-income countries.2 One of the interventions proposed for performance during labour with the aim of reducing neonatal mortality is administration of antenatal corticosteroids.3
The administration of corticosteroids is mainly recommended in women at risk of preterm birth before 34 weeks’ gestation,4–6 but beneficial effects have been described even in late-preterm newborns.7,8 There is evidence that their administration significantly reduces the risk of perinatal death, respiratory distress syndrome (RDS), intraventricular haemorrhage (IVH), necrotising enterocolitis, retinopathy of prematurity, systemic infection,9–11 neurodevelopmental abnormalities and cerebral palsy,12 but most studies have been conducted in first world countries, so the outcomes in developing countries could be different.9,13
The corticosteroids that cross the placental barrier and may be used for this purpose are betamethasone and dexamethasone.5,14–16 Their effect peaks 24–48h after administration,6 but some authors have described the use of incomplete courses or schemes with a short interval between doses with inconsistent results.17
The aim of our study was to measure the frequency of the use of antenatal corticosteroids and estimate their effect on the morbidity and mortality of preterm newborns in a neonatal care unit in western Mexico.
MethodsWe conducted a retrospective cohort study at the Hospital Civil de Guadalajara Dr. Juan I. Menchaca (HCGJIM) in the city of Guadalajara in Jalisco, Mexico. Our hospital provides health care services to the uninsured and low-income population. The department of obstetrics includes the labour and delivery unit (12 beds, 3 operating rooms and 3 delivery rooms) and the inpatient unit (91 beds); the department of neonatology includes 18 neonatal intensive care beds and 57 intermediate care beds.
We selected patients from the cohort of patients documented in the records between January 25, 2016 and August 31, 2017, which include information on the demographic and clinical characteristics, morbidity and mortality of all children born in the hospital (date of birth, sex, gestational age, 5-minute Apgar score, mode of delivery, use of antenatal corticosteroids, death during hospitalisation, cause of death, maternal risk factors for neonatal infection and maternal age).
From this study universe, we selected the newborns delivered before 37 weeks’ gestation that were admitted to the neonatal unit after birth, excluding newborns transferred from other units in the hospital or born outside the period under study. We calculated the sample size needed to identify an OR of 0.5,9 assuming the known proportion of death in hospitalised newborns in our hospital of 12.5%,18 for an alfa of 0.05 and a power of 0.80.
We made a retrospective review of maternal records to obtain data on the prescription and administration of antenatal corticosteroids (type of corticosteroids, dosage, number of doses and latency period; latency period was defined as the number of hours elapsed from administration to the first dose of corticosteroids to the time of delivery). The drugs were prescribed by the gynaecologists-obstetricians of the hospital in compliance with the clinical practice guidelines of the Secretariat of Health (betamethasone or dexamethasone, 2 intramuscular 12mg doses given 12–24h apart).19
We reviewed the health records of the newborns to find out whether they presented any of the following during hospitalisation: RDS, IVH, early-onset neonatal sepsis (EONS), late-onset neonatal sepsis (LONS) and chronic lung disease (CLD).
DefinitionsWe obtained information on RDS, IVH, EONS, LONS and CLD from the patient health records. The physicians in charge of the patient made the diagnoses according to the following definitions:
- -
Respiratory distress syndrome: progressive breathing difficulty from the first minutes of life with increasing oxygen requirements and radiologic findings associated with increased alveolar surface tension.
- -
Intraventricular haemorrhage: bleeding confined to the cerebral ventricles, diagnosis made by ultrasound scan performed by a radiologist and severity graded according to the Papile classification.
- -
Neonatal sepsis: presence of microbial growth in culture of blood or cerebrospinal fluid (CSF) samples from the patient and clinical manifestations suggestive of infection. In cases where the bacterium isolated from blood or CSF was coagulase-negative Staphylococcus, diagnosis required 2 or more positive blood cultures or detection of abnormal white blood cell counts and glucose levels in CSF analysis. Sepsis was classified as early-onset if it manifested in the first 72h of life, and as late-onset otherwise.
- -
Chronic lung disease: oxygen dependency at 36 weeks of postmenstrual age or at hospital discharge in preterm newborns delivered before 32 weeks’ gestation; in patients delivered at 32 weeks’ gestation or later, it was diagnosed if they were oxygen-dependent for more than 28 days post birth.
- -
Cause of death (primary cause): illness or lesion that started the chain of pathological events that led to death. It was assessed by 3 paediatric neonatologists and the cause was assigned if there was agreement in 2 or more of their opinions.
We performed a descriptive analysis of the total sample and then we classified the cohort according to the history of antenatal corticosteroid administration. We summarised qualitative variables as absolute frequencies and quantitative variables as mean, standard deviation (SD) and range. We compared proportions with the chi square test or the Fisher exact test and means with the Student t test or the Fisher exact test. We defined statistical significance as a P-value of less than .05.
We analysed the association between administration of antenatal corticosteroids and the dependent variables by means of the chi square test, followed by a logistic regression analysis in which we adjusted the groups by birth weight and gestational age. We performed these analyses for the overall sample and for the subset of patients born before 34 weeks’ gestation. To analyse the impact of corticosteroids across time, we performed a survival analysis, plotting Kaplan–Meier curves and using the log-rank test for hypothesis testing.
We used the IBM SPSS Statistics software version 20 for the analysis. The project was approved by the ethics and research committees of the HCGJIM under file number 0184/17.
ResultsDuring the period under study, the unit admitted 1083 preterm newborns, 53.3% (n=577) male, 46.4% (n=503) female, and 3 with ambiguous genitalia. Their mean gestational age was 33.4 weeks (maximum, 36.6; minimum, 24; SD, 2.73); 46.6% (n=505) were born before 34 weeks’ gestation and 25.3% (n=274) at or before 32 weeks’ gestation. The mean birth weight was 1765.1g (maximum, 3775; minimum, 410; SD, 606.8). Of all newborns, 70.7% (n=766) were born by caesarean section.
Antenatal corticosteroids were given to 42% of the sample (455/1083): dexamethasone to 76.9% (n=350) and betamethasone to 23.1% (n=105); the full course of treatment was only completed in 61.3% of these patients (279/455). Of all newborns delivered before 34 weeks’ gestation, 53.7% had been exposed to corticosteroids (271/505).
In our sample, 35% (n=379) of newborns developed RDS, 4.4% (n=48) EONS and 10.7% (n=116) LONS. A transfontanellar ultrasound was performed in 83.8% (n=908), which led to identification of IVH in 15.1% (137/908), the severity of IVH was grade i in 33.5%, ii in 27%, iii in 23.4% and iv in 16.1%.
The length of stay was of 28 days or longer in 29.6% of the sample (n=321); within this subset, 51.4% (165/321) had CLD. We ruled out CLD in all preterm newborns discharged before 28 days post birth, as they were all discharged home without a prescription for supplemental oxygen.
The mortality in our sample was 22.3% (n=242), and the leading causes of death were infection (28.9%; n=70), respiratory distress syndrome (23.6%; n=57), congenital malformations or genetic disorders (20.7%; n=50) and intraventricular haemorrhage (8.7%; n=21).
When we compared gestational age subgroups (<28 weeks [n=50], 28–32 weeks [n=224], 32.1–33.9 weeks [n=231] and ≥34 weeks [n=578]), we found significant differences in mortality (94%, 44.2%, 17.3%, 9.7%; P<.001); respiratory distress syndrome (76%, 77.2%, 38.5%, 13.7%; P<.001); chronic lung disease at 28 days post birth or later (42.9%, 56%, 21.2%, 7.6%; P<.001) and intraventricular haemorrhage (66.7%, 35.7%, 9.3%, 6.1%; P<.001).
When we compared the clinical and demographic characteristics based on the administration of antenatal corticosteroids, we found that patients that received this intervention had a lower gestational age and weight at birth and were more likely to have been delivered by caesarean section and have mothers with risk factors for neonatal infection (urinary tract infection, chorioamnionitis, premature rupture of membranes or fever during labour) (Table 1).
Clinical and demographic characteristics of hospitalised preterm newborns based on the prenatal administration of corticosteroids.
The analysis of the frequency of RDS, EONS, LONS, IVH, CLD and death by antenatal corticosteroid use did not reveal a protective effect of this intervention, regardless of the administered dose or schedule; in fact, we found an increased incidence of RDS, IVH and in patients exposed to corticosteroids (Table 2). When we analysed the outcomes of this intervention adjusted for gestational age and birth weight for the total sample and the subgroup born before 34 weeks’ gestation, we only found a reduction in mortality (37%) in newborns delivered before 34 weeks’ gestation (Table 3).
Morbidity and mortality in hospitalised preterm newborns based on the use of prenatal corticosteroids.
| Prenatal corticosteroidsn=455 | No corticosteroidsn=628 | Pa | |
|---|---|---|---|
| Respiratory distress syndrome (%) | 43.5 | 28.8 | <.001 |
| Early-onset neonatal sepsis (%) | 5.5 | 3.7 | .15 |
| Late-onset neonatal sepsis (%) | 12.1 | 9.7 | .21 |
| Intraventricular haemorrhage (%) | 17.9 | 12.9 | .04 |
| Bronchopulmonary dysplasia (%) | 24.0 | 15.2 | .001 |
| Death (%) | 25.3 | 20.2 | .04 |
Morbidity and mortality in hospitalised preterm newborns and in the subgroup of newborns delivered before 34 weeks’ gestation by use of prenatal corticosteroids (adjusted for gestational age and birth weight using logistic regression).
| Total samplen=1083OR (95% CI) | Gestational age <34 weeksn=505OR (95% CI) | |
|---|---|---|
| Respiratory distress syndrome | 1.16 (0.85–1.57) | 1.25 (0.84–1.87) |
| Early-onset neonatal sepsis | 1.53 (0.84–2.77) | 1.05 (0.44–2.48) |
| Late-onset neonatal sepsis | 1.09 (0.73–1.62) | 1.08 (0.66–1.77) |
| Intraventricular haemorrhagea | 0.81 (0.53–1.23) | 0.76 (0.47–1.25) |
| Bronchopulmonary dysplasiab | 0.94 (0.59–1.50) | 1.02 (0.59–1.80) |
| Death | 0.73 (0.52–1.04) | 0.63 (0.40–0.98) |
In the group of patients exposed to corticosteroids, 51.9% (236/455) had latency periods of 24h or longer, and only 32.9% (150/455) of 48h or longer; in 32 patients, the health records did not have the information necessary to estimate the duration of latency. When we compared patients that received corticosteroids with a latency of 48h or greater and patients that did not receive corticosteroids, we found a decreased risk of death (60%) in patients that received corticosteroids born before 34 weeks’ gestation, without other significant effects on the remaining outcomes (Table 4); we also found a protective trend that was not significant on the incidence of IVH of grades iii and iv in patients born before 34 weeks’ gestation (OR, 0.35; 95% CI, 0.11–1.07; P=.06). When we analysed the dependent variables in relation to a latency period of 24h or more, we found no differences in outcomes when we compared patients treated with corticoids and untreated patients.
Morbidity and mortality in hospitalised preterm newborns and in the subgroup of newborns delivered before 34 weeks’ gestation by use of prenatal corticosteroids with a latency period of ≥48h (adjusted for gestational age and birth weight using logistic regression).
| Total samplen=778aOR (95% CI) | Gestational age <34 weeksn=310aOR (95% CI) | |
|---|---|---|
| Respiratory distress syndrome | 0.89 (0.57–1.40) | 0.87 (0.48–1.57) |
| Early-onset neonatal sepsis | 1.75 (0.78–3.92) | 0.61 (0.13–2.87) |
| Late-onset neonatal sepsis | 1.41 (0.83–2.40) | 1.62 (0.84–3.12) |
| Intraventricular haemorrhageb | 0.66 (0.35–1.24) | 0.65 (0.31–1.37) |
| Bronchopulmonary dysplasiac | 1.0 (0.53–1.91) | 1.19 (0.56–2.54) |
| Death | 0.59 (0.35–1.03) | 0.40 (0.20–0.80)a |
We did not find significant differences in morbidity and mortality between patients with latency periods of 48h or longer treated with betamethasone vs dexamethasone, although there were few patients in this subset.
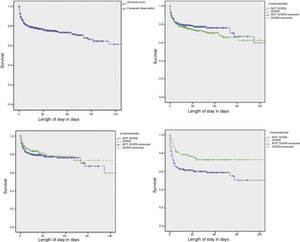
We used Kaplan–Meier survival analysis to assess the effect of antenatal corticosteroids in the survival of preterm newborns admitted to the HCGJIM, and found that the intervention was most beneficial in infants born before 34 week's gestation with latency periods of 48h or longer (Fig. 1).
Survival of hospitalised preterm newborns by administration of antenatal corticosteroids, with a latency period of 48 or more hours, and in the subset of newborns born before 34 weeks’ gestation.
Survival of hospitalised preterm newborns (n=1083).
Preterm newborns exposed to corticosteroids vs not exposed (n=1083), P=.30 (log rank).
Preterm newborns treated with corticosteroids with a latency period ≥48h vs not treated with corticosteroids (n=778), P=.34 (log-rank test).
Preterm newborns born before 34 weeks that received corticosteroids with a latency period ≥48h vs preterm newborns <34 weeks not treated with corticosteroids (n=310), P=.009 (log-rank test).
It is estimated that 10% of infants worldwide are born preterm,20 and that this group has a greater morbidity and mortality compared to full-term infants.18 In Mexico, based on this proportion and the number of births recorded between 2014 and 2016,21 there would be an average of 237000 preterm births per year.
Since 1972, there has been evidence that in preterm newborns, antenatal exposure to corticosteroids reduced the risk of neonatal death through their acceleration of foetal lung maturation,4,9 yet to date adherence to the recommendation of this intervention has been variable. In our study, we found that fewer than 50% of preterm newborns had been exposed to antenatal corticosteroids, and that only 25.8% had received the full course of steroids and only 13.8% the full course with latency periods of 48h or longer.
A trial conducted by Berrueta et al.22 in low- and middle-income countries revealed that despite the existence of guidelines for antenatal corticosteroid therapy, this intervention was only performed in 2% to 44% of mothers of preterm babies, with the lowest frequencies found in African countries.
Aleman et al.23 found that in Mexico, 70% of women in preterm labour received antenatal corticosteroids, and that this proportion was lower in the group of mothers with comorbidities (39–62.2%). In addition, the frequency of antenatal corticosteroid therapy was significantly higher in El Salvador and Ecuador.
Roberts et al. found that antenatal steroids, compared to placebo, decreased the risk of perinatal death (RR, 0.72, 95% CI 0.58 to 0.89), respiratory distress syndrome (RR, 0.66; 95% CI, 0.56–0.77), intraventricular haemorrhage (RR, 0.55; 95% CI: 0.40–0.76), and systemic infection in the first 48h post birth (RR, 0.60; 95% CI: 0.41–0.88). They concluded that there was sufficient evidence to recommend this intervention; however, given that most studies have been conducted in developed countries, there is little evidence on the outcomes of this intervention in low-income countries, where the incidence of infection is high.9
Previous studies suggest that the greatest benefit of antenatal corticosteroids is achieved when birth occurs 24–48h after the administration of the first dose.6,24 In our study, we found a significant decrease in the risk of mortality (37%) associated with the use of antenatal corticosteroids in the group of patients born before 34 weeks’ gestation, and when we analysed the specific subset with a latency period of 48 or more hours, we found an even greater decrease in risk (60%); we did not find statistically significant differences in the incidence of RDS, EONS, LONS, IVH or CLD.
It is likely that in our patients in the HCGJIM, the limited impact on neonatal mortality was due to the low proportion of preterm newborns that had been subject to this intervention with latency periods greater than 24–48h, although it would be necessary to evaluate the effect of other interventions, such as prenatal care, the treatment of maternal comorbidities, the appropriate delivery of neonatal intensive care, the administration of surfactant and the prophylactic use of caffeine.
When we assessed the effecs of the intervention by gestational age groups without adjusting for any other variables, we found the greatest benefit in newborns delivered between 32 and 33.9 weeks’ gestation (n=231) in terms of a reduction in neonatal mortality (OR, 0.41; 95% CI, 0.20–0.85). We did not find significant changes in mortality in any subgroup. Our findings were consistent with those of Gyamfi-Bannerman et al.,8 who did not find changes in mortality in late preterm newborns in association with exposure to antenatal corticosteroids; however, these authors did observe a reduction in the incidence of respiratory disease.
Norman et al.25 evaluated the effect of antenatal corticosteroids based on the time elapsed from administration of the first dose to birth and found a stronger impact on mortality when the latency period was greater than 24h (OR, 0.5; 95% CI, 0.4–0.6). Nevertheless, their findings suggested that a latency period of 3h can already reduce risk (26%). The authors did not find any decreases in morbidity in patients with latency periods greater than 7 days.
In a study of low- and middle-income countries, Althabe et al.26 did not find a decrease in the risk of neonatal death (RR, 0.96, 95% CI, 0.87–1.06) after increasing the frequency of administration of antenatal corticosteroids from 10% to 45%, but they found an increase in mortality in exposed patients of 12.7% (from 23.9 deaths per 1000 live births to 27.4 deaths per 1000 live births; P=.01). There was also an increase in the incidence of maternal infection (RR, 1.45; 95% CI, 1.33–1.58).
A secondary analysis by these authors suggested that the increase in mortality was associated with deaths due to bacterial infection (RR, 1.36; 95% CI, 1.12–1.65).27 In our patients at the HCGJIM, we found that the risk of death due to bacterial infection increased as gestational age decreased (RR, 1.17; 95% CI, 1.09–1.26), and found no correlation with the use of antenatal corticosteroids (RR, 1.34; 95% CI, 0.81–2.20).
Similar to what we found in our patients, Sasaki et al.10 described a frequency of corticosteroid use in preterm newborns delivered before 34 weeks’ gestation of 40.6% and a significant reduction in neonatal mortality, with no associated reduction in the incidence of RDS. When they analysed outcomes based on mode of delivery, they found that administration of antenatal corticosteroids was associated with a reduced risk of RDS in newborns product of vaginal deliveries. In our study at the HCGJIM, when we adjusted the analysis of outcomes based on mode of delivery we found no changes in the risk of RDS (OR, 1.12; 95% CI, 0.82–1.53).
The main limitation of this study is the low number of patients that completed the full course of antenatal corticosteroids or with latency periods of 48h or greater. Given that we only included patients born preterm that required admission at birth, it is possible that there was selection bias; however, we ought to note that all patients born before 34 weeks’ gestation or with low birth weight were admitted to the unit.
Conflicts of interestThe authors have no conflicts of interest to declare
Please cite this article as: Pérez-Ramírez RO, Lona-Reyes JC, Ochoa-Meza CA, Gómez-Ruiz LM, Ramos-Gutiérrez RY, Camarena-Pulido EE, et al. Morbimortalidad neonatal en un entorno de baja adherencia a corticosteroides prenatales. An Pediatr (Barc). 2019;91:105–111.