It is estimated that about 70 million people all over the world suffer from epilepsy, half of which are children, in whom the prevalence is around 0.5–0.8%. Although there are several therapies, the treatment of epilepsy is based mainly on drugs, which, depending on the year of coming onto the market are classified as first, second, or third generation. In this article, a description is presented on the main characteristics of the latest generation of anti-epileptic drugs (lacosamide, eslicarbazepine acetate, brivaracetam, perampanel, retigabine, everolimus and cannabidiol). These, with the exception of retigabine (is not yet on the market), are considered safe and effective in the paediatric population. Everolimus and cannabidiol have very specific indications (tuberous sclerosis, Dravet syndrome, and Lennox Gastaut syndrome), while the rest are indicated in the management of seizures of focal origin in children from 4 years-old. These new molecules have been developed in order to provide a pharmaceutical profile and tolerance superior to the previously available drugs, and it is forecast that as their use increases, their true potential and profile will widen. Furthermore, for the first time in Paediatric Epileptology, the extrapolation of the efficacy data in adults have been used (together with specific safety and pharmacokinetic studies in the paediatric population), in order to speed up their approval for use in the child population.
Se estima que unos 70 millones de personas padecen epilepsia a nivel mundial de los cuales más de la mitad son niños, en los que la prevalencia estimada se sitúa en torno al 0,5-0,8%. Aunque existen diversas terapias, el tratamiento de la epilepsia se basa mayoritariamente en fármacos, que en función de su año de comercialización se clasifican como de primera, segunda o tercera generación. En el presente artículo se revisan las principales características de los fármacos antiepilépticos de última generación (lacosamida, acetato de eslicarbazepina, brivaracetam, perampanel, retigabina, everolimus y cannabidiol) que, con excepción de la retigabina (ya no está comercializada), se consideran seguros y efectivos en población pediátrica. El everolimus y el cannabidiol tienen indicaciones muy concretas (esclerosis tuberosa, síndrome de Dravet y síndrome de Lennox Gastaut) mientras que el resto están indicados en el manejo de crisis de origen focal en niños a partir de 4 años. Estas nuevas moléculas han sido desarrolladas para aportar un perfil farmacocinético y de tolerancia superior a los fármacos previamente disponibles y es previsible que a medida que aumente su uso, se vaya perfilando y ampliando su verdadero potencial. Además, por primera vez en epileptología pediátrica, se ha utilizado la extrapolación de datos de efectividad en adultos (junto con estudios de seguridad y farmacocinética específicos en población pediátrica), para acelerar la aprobación de uso en población infantil.
It is estimated that approximately 70 million people worldwide suffer from epilepsy, of who more than half are of paediatric age.1–3 Thus, this is a relatively frequent neurologic disorder in children that impairs the quality of life of patients and their families, poses a substantial burden to society and with a significant economic impact on health care systems.1
The term epilepsy encompasses a broad and heterogeneous range of disorders and syndromes with a high variability in terms of the aetiology, severity and course of disease. All of the above has significant implications in terms of diagnosis and management, which, depending on the geographical area, the complexity of the case and the availability of health care providers, are carried out at different levels of specialization (general practitioners, general paediatricians, neurologists, paediatric neurologists or paediatric epileptologists).
Epilepsy is a disease that is clearly influenced by the level of development of a society, as both its incidence and prevalence are higher in developing countries (it is estimated that between 75% and 80% of new cases occur in these regions).3,4 The incidence of seizures is highest in the first year of life (100 cases/100000) and decreases with age until reaching a rate of approximately 20 cases/100000 in adolescence. The estimated overall prevalence in the paediatric age group (<16 years) is of 0.5%–0.8%,5,6 with a predominance of focal forms (59%–63%) and a lesser frequency of generalised epilepsy (12%–29%), although in 20% of cases the classification may vary in the same patient during the course of the disease.5,7
In children as well as adults, the treatment of epilepsy is mostly based on the use of drugs (either in monotherapy or combined therapy), and the remaining approaches (surgery, neuromodulation, ketogenic diet) are used much less frequently. In addition, most patients managed with nonpharmacological approaches require drugs as adjuvant treatment to achieve optimal seizure control.
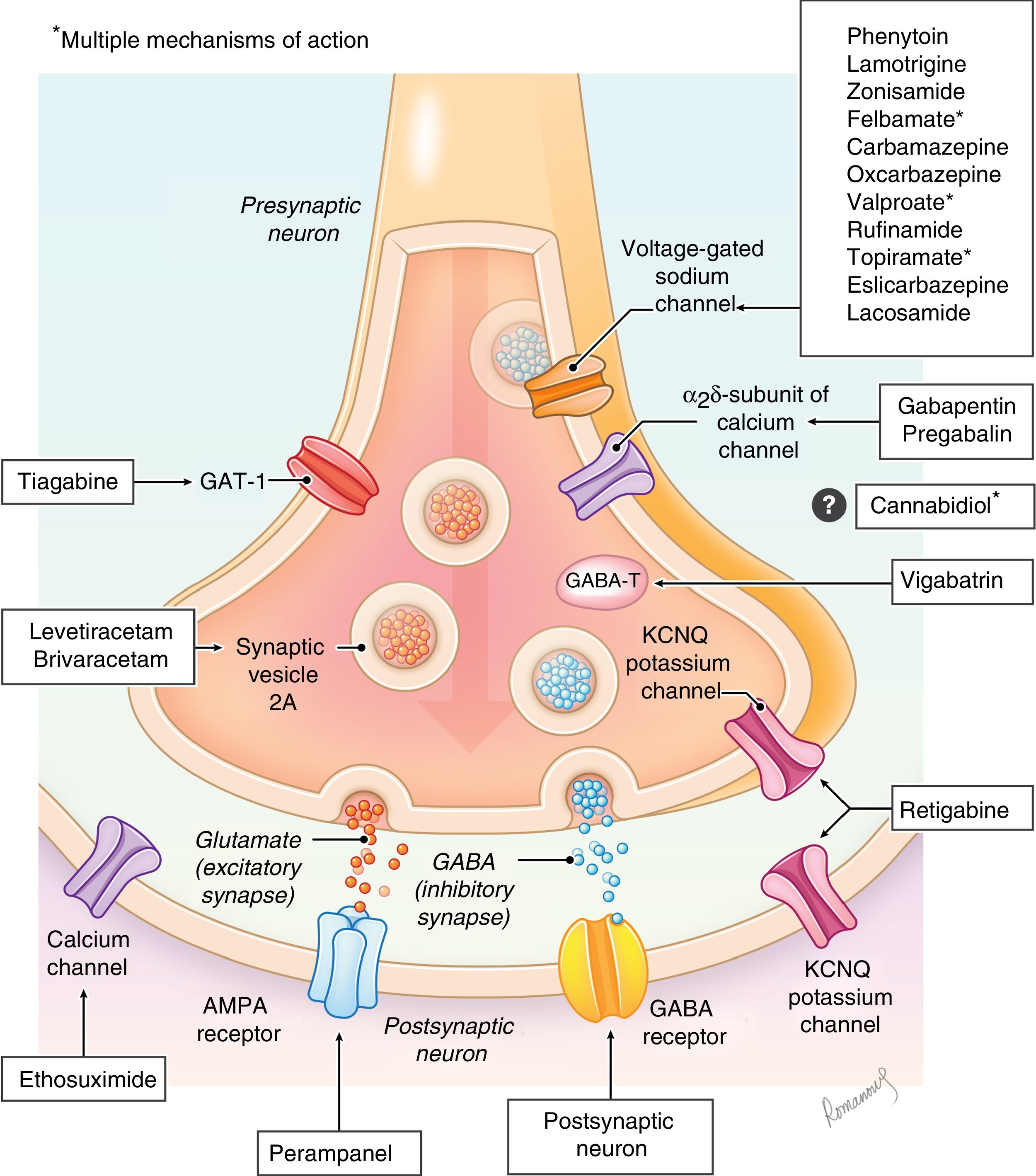
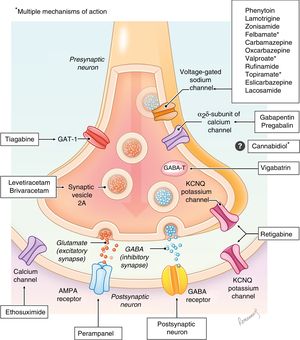
Antiepileptic drugs are molecules that act at different levels of the synapse with the aim of modifying the excitatory and/or inhibitory responses through different mechanisms (sodium or calcium channels, GABA receptors, glutamate, etc). Their mechanisms of action are diverse, and are not even fully understood in some of the drugs (Fig. 1), and there are excellent review articles on the subject.8–10 At present, there are more than 25 drugs classified based on the year they were authorised for distribution as first-, second- or third-generation (Table 1).11,12
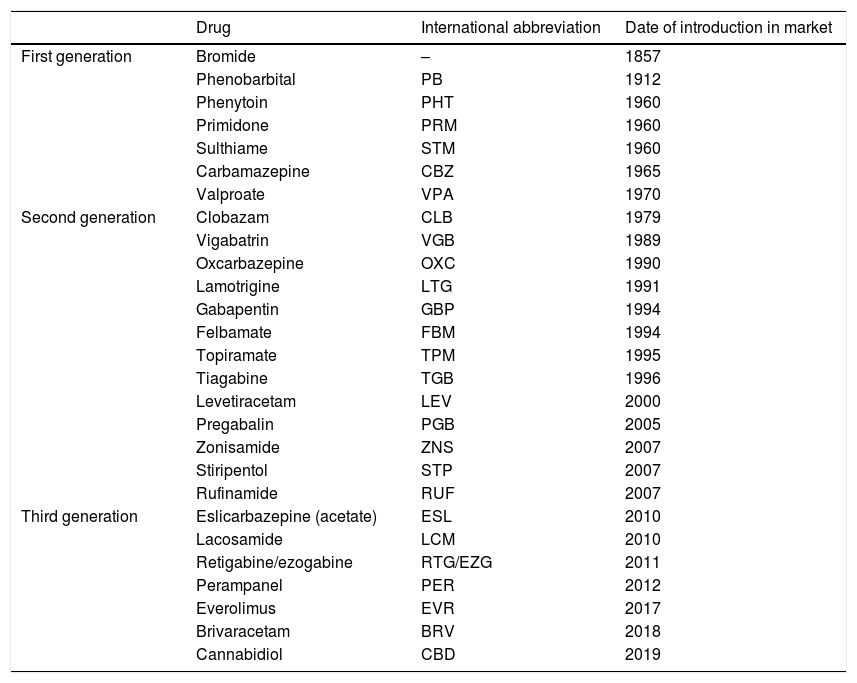
Main antiepileptic drugs and year of introduction in Europe (authorization of the European Medicines Agency), in chronological order.
| Drug | International abbreviation | Date of introduction in market | |
|---|---|---|---|
| First generation | Bromide | – | 1857 |
| Phenobarbital | PB | 1912 | |
| Phenytoin | PHT | 1960 | |
| Primidone | PRM | 1960 | |
| Sulthiame | STM | 1960 | |
| Carbamazepine | CBZ | 1965 | |
| Valproate | VPA | 1970 | |
| Second generation | Clobazam | CLB | 1979 |
| Vigabatrin | VGB | 1989 | |
| Oxcarbazepine | OXC | 1990 | |
| Lamotrigine | LTG | 1991 | |
| Gabapentin | GBP | 1994 | |
| Felbamate | FBM | 1994 | |
| Topiramate | TPM | 1995 | |
| Tiagabine | TGB | 1996 | |
| Levetiracetam | LEV | 2000 | |
| Pregabalin | PGB | 2005 | |
| Zonisamide | ZNS | 2007 | |
| Stiripentol | STP | 2007 | |
| Rufinamide | RUF | 2007 | |
| Third generation | Eslicarbazepine (acetate) | ESL | 2010 |
| Lacosamide | LCM | 2010 | |
| Retigabine/ezogabine | RTG/EZG | 2011 | |
| Perampanel | PER | 2012 | |
| Everolimus | EVR | 2017 | |
| Brivaracetam | BRV | 2018 | |
| Cannabidiol | CBD | 2019 |
The management of epilepsy in the paediatric age group has specific characteristics, since many of the forms of epilepsy in this population are different from those found in adults (including most of the epileptic syndromes), and so does the management of antiepileptic drugs and the potential adverse events or sequelae derived from their use, as they differ from those in adults.11 On the other hand, there are also similarities with the management in adults, specifically, the disease progression and response to treatment of focal-onset seizures is similar in children aged 2 or more years and in adult patients, which makes it possible to extrapolate data from adult studies to expedite authorization of new drugs for use in children.13
Obtaining authorization for use of new antiepileptic drugs in the paediatric population usually takes years, which encourages off-label use in this age group. This results in a lack of safety for the patient (absence of validated data on the dosage, safety and tolerability) and ethical and legal problems for the prescriber.13 Extrapolation, a term initially established by the Food and Drug Administration (FDA) in 1994 and now used and validated at the international level, may be used to shorten the time elapsed between authorization of a drug for use in adults and its authorization for use in children.13,14 In paediatrics, the aim of extrapolation is to guarantee the efficient use of data obtained in adults in the development of drugs for use in the paediatric population. Extrapolation is defined as “extending information and conclusions available from studies in one or more subgroups of the patient population (source population(s)), or in related conditions or with related medicinal products, in order to make inferences for another subgroup of the population (target population), or condition or product, thus reducing the amount of, or general need for, additional evidence generation (types of studies, design modifications, number of patients required) needed to reach conclusions.”13,15 In order to extrapolate information on drugs, several conditions must be assumed to be true, the most important of which are: (a) that the course of disease is similar in adults children, (b) that the response to treatment is similar in adults and children, and (c) that the response to exposure to the drug is similar in both populations.13,15
In epilepsy, extrapolation cannot be performed generally, as there are significant differences between children and adults in its aetiology, types of epileptic syndromes and natural history, but similarities have been established in focal-onset seizures between children aged 4 or more years and adults.13 Nevertheless, it is important to take into account that while it is possible to assume similarities in efficacy, it is not possible to extrapolate data on other aspects such as dosage, pharmacokinetics or safety, and therefore studies in the paediatric age group continue to be necessary.
In this study, we will merely review the main novelties in pharmacological management of epilepsy in the paediatric age group. This last generation of drugs is the first to include molecules whose effectiveness in children has not been demonstrated through specific double-blind randomised controlled clinical trials, but based on the extrapolation from data obtained in adult studies (eslicarbazepine acetate, brivaracetam, lacosamide, perampanel).
Eslicarbazepine acetate (ESL)Eslicarbazepine acetate is a third-generation drug in the carboxamide family (CBZ, OXC) that acts by blocking voltage-gated sodium channels, resulting in a slow inactivation of neuron excitability. It was initially authorised for use in adults in Europe by the EMA in 2010 and later in 2013 in the United States by the FDA for adjuvant treatment of focal-onset seizures with or without secondary generalization. This indication was subsequently extended to include use as monotherapy. Since 2017 it has been authorised for use in the paediatric population for adjuvant treatment of focal-onset seizures with or without secondarily generalised seizures in children aged 4 or more years (FDA) or children aged 6 or more years (EMA), making it the first antiepileptic drug approved for use in children based on extrapolation. The main advantages of ESL are its administration as a single daily dose (which facilitates adherence to treatment), the absence of significant interactions with other drugs and a favourable safety profile, as no significant changes in haematology or chemistry parameters have been described. In this regard, hyponatraemia tends to be less frequent in comparison with OXC.11,16 The adverse effects seem to be dose-dependent and are presented in Table 2. This drug does not seem to cause cognitive or behavioural adverse effects in children.17
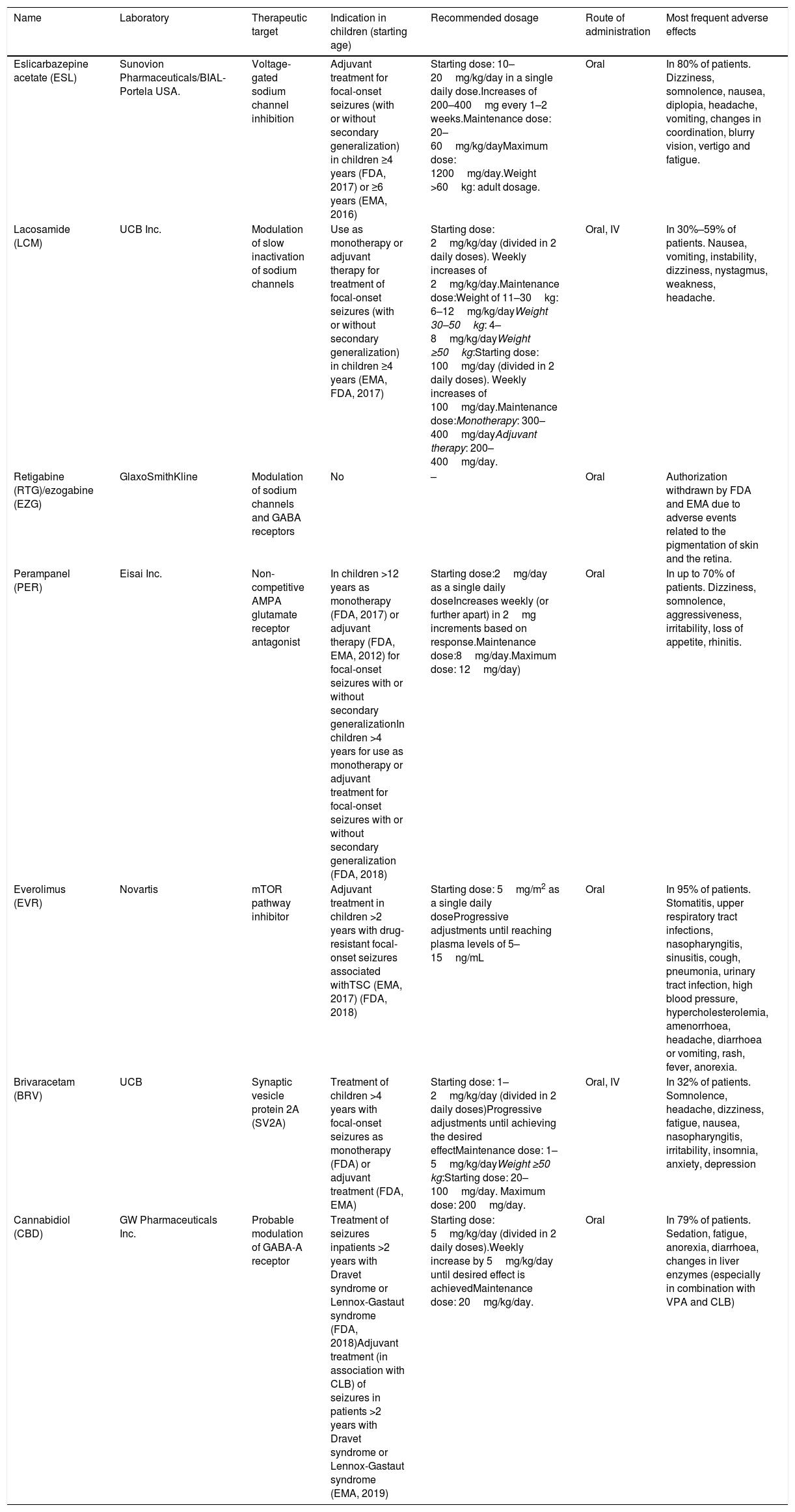
Main characteristics of third-generation AEDs used in the paediatric population.
| Name | Laboratory | Therapeutic target | Indication in children (starting age) | Recommended dosage | Route of administration | Most frequent adverse effects |
|---|---|---|---|---|---|---|
| Eslicarbazepine acetate (ESL) | Sunovion Pharmaceuticals/BIAL-Portela USA. | Voltage-gated sodium channel inhibition | Adjuvant treatment for focal-onset seizures (with or without secondary generalization) in children ≥4 years (FDA, 2017) or ≥6 years (EMA, 2016) | Starting dose: 10–20mg/kg/day in a single daily dose.Increases of 200–400mg every 1–2 weeks.Maintenance dose: 20–60mg/kg/dayMaximum dose: 1200mg/day.Weight >60kg: adult dosage. | Oral | In 80% of patients. Dizziness, somnolence, nausea, diplopia, headache, vomiting, changes in coordination, blurry vision, vertigo and fatigue. |
| Lacosamide (LCM) | UCB Inc. | Modulation of slow inactivation of sodium channels | Use as monotherapy or adjuvant therapy for treatment of focal-onset seizures (with or without secondary generalization) in children ≥4 years (EMA, FDA, 2017) | Starting dose: 2mg/kg/day (divided in 2 daily doses). Weekly increases of 2mg/kg/day.Maintenance dose:Weight of 11–30kg: 6–12mg/kg/dayWeight 30–50kg: 4–8mg/kg/dayWeight ≥50kg:Starting dose: 100mg/day (divided in 2 daily doses). Weekly increases of 100mg/day.Maintenance dose:Monotherapy: 300–400mg/dayAdjuvant therapy: 200–400mg/day. | Oral, IV | In 30%–59% of patients. Nausea, vomiting, instability, dizziness, nystagmus, weakness, headache. |
| Retigabine (RTG)/ezogabine (EZG) | GlaxoSmithKline | Modulation of sodium channels and GABA receptors | No | – | Oral | Authorization withdrawn by FDA and EMA due to adverse events related to the pigmentation of skin and the retina. |
| Perampanel (PER) | Eisai Inc. | Non-competitive AMPA glutamate receptor antagonist | In children >12 years as monotherapy (FDA, 2017) or adjuvant therapy (FDA, EMA, 2012) for focal-onset seizures with or without secondary generalizationIn children >4 years for use as monotherapy or adjuvant treatment for focal-onset seizures with or without secondary generalization (FDA, 2018) | Starting dose:2mg/day as a single daily doseIncreases weekly (or further apart) in 2mg increments based on response.Maintenance dose:8mg/day.Maximum dose: 12mg/day) | Oral | In up to 70% of patients. Dizziness, somnolence, aggressiveness, irritability, loss of appetite, rhinitis. |
| Everolimus (EVR) | Novartis | mTOR pathway inhibitor | Adjuvant treatment in children >2 years with drug-resistant focal-onset seizures associated withTSC (EMA, 2017) (FDA, 2018) | Starting dose: 5mg/m2 as a single daily doseProgressive adjustments until reaching plasma levels of 5–15ng/mL | Oral | In 95% of patients. Stomatitis, upper respiratory tract infections, nasopharyngitis, sinusitis, cough, pneumonia, urinary tract infection, high blood pressure, hypercholesterolemia, amenorrhoea, headache, diarrhoea or vomiting, rash, fever, anorexia. |
| Brivaracetam (BRV) | UCB | Synaptic vesicle protein 2A (SV2A) | Treatment of children >4 years with focal-onset seizures as monotherapy (FDA) or adjuvant treatment (FDA, EMA) | Starting dose: 1–2mg/kg/day (divided in 2 daily doses)Progressive adjustments until achieving the desired effectMaintenance dose: 1–5mg/kg/dayWeight ≥50 kg:Starting dose: 20–100mg/day. Maximum dose: 200mg/day. | Oral, IV | In 32% of patients. Somnolence, headache, dizziness, fatigue, nausea, nasopharyngitis, irritability, insomnia, anxiety, depression |
| Cannabidiol (CBD) | GW Pharmaceuticals Inc. | Probable modulation of GABA-A receptor | Treatment of seizures inpatients >2 years with Dravet syndrome or Lennox-Gastaut syndrome (FDA, 2018)Adjuvant treatment (in association with CLB) of seizures in patients >2 years with Dravet syndrome or Lennox-Gastaut syndrome (EMA, 2019) | Starting dose: 5mg/kg/day (divided in 2 daily doses).Weekly increase by 5mg/kg/day until desired effect is achievedMaintenance dose: 20mg/kg/day. | Oral | In 79% of patients. Sedation, fatigue, anorexia, diarrhoea, changes in liver enzymes (especially in combination with VPA and CLB) |
Lacosamide is a functionalised amino acid that acts on the neuron by enhancing selective slow inactivation of voltage-gated sodium channels. It does not affect rapid inactivation, so it is believed to modulate the pathological neuronal hyperexcitability without altering the physiological function of the cell.18 It was first authorised for use in adults aged more than 17 years by the FDA (2008) for adjuvant treatment of focal-onset seizures with or without secondary generalization. Since 2017, it has been authorised by the EMA and the FDA for use as monotherapy or add-on treatment in children from age 4 years. Lacosamide has the advantage of being an effective, safe and well-tolerated drug in the paediatric population, with little interaction with other drugs and that may delivered by both the enteral and parenteral routes. Furthermore, based on case series published in the past 5 years, in the future it could be considered a safe and effective treatment for status epilepticus and refractory status epilepticus in the paediatric age group.19Table 2 presents the most frequent adverse events, revealing a favourable neurotoxicity profile: depression in 9.5%, memory changes in 7.4% and other forms of cognitive impairment in 4.3%.11,20
Retigabine (RTG)/ezogabine (EZG)Retigabine (international nonproprietary name) or ezogabine (United States adopted name) exhibits a unique mechanism of action among antiepileptic drugs, as it acts by activating KCNQ2-5 potassium channels, although it also modulates GABA receptors.4,10 This drug was authorised by the FDA and the EMA in 2011 for treatment of focal epilepsy with or without secondarily generalised seizures in adults. Later on, authorization was withdrawn by both agencies (FDA in 2017, EMA in 2018) upon request from manufacturer due to adverse effects involving skin pigmentation and the retina, so it is not currently available. It was never authorised for use in the paediatric population, and there is a single article in the paediatric literature describing successful treatment of a girl aged 8 years with ring chromosome 20 syndrome. The region where this fusion occurs most frequently is q13.3, where the KCNQ2 gene is located.21
Perampanel (PER)Perampanel is one of the drugs authorised most recently for treatment of epilepsy in children (FDA 2018), although it has been authorised for treatment of adults and children aged 12 or more years since 2012. It acts as a highly selective non-competitive AMPA glutamate receptor antagonist (localized at excitatory synapses, post-synaptically), which makes its mechanism of action unique among the antiepileptic drugs. The inhibition results from the modulation of fast excitatory synaptic transmission.22,23 In adults and children aged more than 12 years, it is indicated for treatment of focal-onset seizures with or without secondary generalization (FDA and EMA, 2012) and of generalised tonic-clonic seizures (EMA and FDA, 2015). In 2018, the FDA expanded the indication to children aged 4 or more years with focal-onset seizures with or without secondary generalization based on extrapolated data after performance of safety and pharmacokinetic studies. Authorization in this age group by the EMA is still pending.13 Furthermore, several studies in children and adolescents have demonstrated its effectiveness in the treatment of focal-onset seizures.22 The main advantages of PER are the low incidence of cognitive side effects, its route of administration, the single daily dose that facilitates adherence to treatment and its favourable risk/benefit ratio. Adverse effects are more frequent in children aged more than 12 years compared to younger children, and they have been described in up to 70% of patients. Psychiatric changes are among the most significant adverse effects it causes (Table 2), including aggressiveness and irritability, which seem to be dose-dependent and have been described in 8.2% to 14% of treated children. They are most frequent in the first 6 weeks of treatment and in patients with psychiatric comorbidities, such as ADHD or personality disorders.10,22
Everolimus (EVR)Everolimus is a drug that in paediatric neurology is mainly used for management of diseases secondary to tuberous sclerosis complex (TSC), a neurocutaneous syndrome with a low prevalence (1 in 6000 births) that is, however, characterised by significant morbidity involving multiple systems and specifically the central nervous system. It is an inhibitor of complex 1 of mTOR (the mammalian target of rapamycin) indicated in patients with TSC for management of subependymal giant cell astrocytoma (SEGA), renal angiomyolipomas and epilepsy. Epilepsy is the most frequent disorder found in patients with TSC (85% of patients), usually with onset in the first year of life (2 out of 3 cases), manifesting either with focal-onset seizures or epileptic spasms. In more than 60% of patients, the seizures are drug-resistant.24 Evidence of its usefulness as an anticonvulsant first emerged during clinical trials of EVR for treatment of SEGA.25,26 Later on, based on the findings of the EXIST-3 trial (an international prospective multicentre double-blind randomised controlled study, n=366),27) the EMA (2017) and the FDA (2018) authorise the indication for use as an adjuvant antiepileptic drug in adults and children aged at least 2 years with drug-resistant focal-onset seizures associated with TSC. Treatment with EVR needs to be adjusted as needed while monitoring its plasma levels, and is likely to produce adverse events (Table 2), with stomatitis being the complication that most frequently leads to a dose reduction or discontinuation of the drug.11,27
Brivaracetam (BRV)Brivaracetam is a modified analogue of levetiracetam (LEV), so it has a similar mechanism of action through binding to synaptic vesicle protein 2A (SV2A), although BRV has more selective binding and a 15- to 30-fold higher binding affinity than LEV.10,28 Its also exhibits partial inhibition of voltage-gated sodium channels. Brivaracetam was first authorised for adjuvant treatment of focal-onset seizures in patients aged 16 or more years in 2016 (FDA, EMA), with its indications later expanding to use as monotherapy (2017) and finally to use in the paediatric population aged 4 or more years (2018) based on extrapolation.
The main advantages of BRV is that it is a safe and effective drug that can be administered enterally or parenterally and that it is very promising due to the high effectiveness it has exhibited against different types of seizures in animal models.13 Its adverse effects are infrequent and are presented in Table 2. We ought to mention that it seems to have fewer adverse behavioural effects compared to LEV.29 Its drawbacks is that it has exhibited more drug-drug interactions compared to LEV, as it can alter the function, and its function be altered by, other hepatic enzyme inducers.10
Cannabidiol (CBD)Cannabidiol is a molecule obtained from the cannabis plant, but unlike tetrahydrocannabinol (THC) it does not interact with cannabinoid receptor B1, so it has antiepileptic effects but no psychoactive effects.30 Its mechanism of action is not fully understood, but it is hypothesised that it may involve an increase in GABA activity through the modulation of the GABA-A receptor, which in turn could regulate intracellular calcium flux through various receptors (TRPV, VDAC, GPR55), and that it may have a mild anti-inflammatory effect mediated by adenosine.10,31 Recently, the FDA (2018) and the EMA (2019) have added the indication of management of seizures in patients aged 2 or more years with Dravet syndrome or Lennox-Gastaut syndrome. The efficacy of CBD was proven in 3 double-blind randomised controlled trials conducted in the paediatric and adult populations,30,32,33 although there is also evidence from other studies suggesting that it may be effective in other forms of epilepsy and epileptic syndromes.34,35 Its main advantage is that it has proven effective in 2 of the epileptic syndromes that are most difficult to control. Its main drawbacks are that it frequently produces adverse events (Table 2), that it interacts with several other antiepileptic drugs and that it may cause hepatotoxicity, especially when used in combination with VPA or VPA and CLB, which requires close monitoring of laboratory parameters (Table 3).
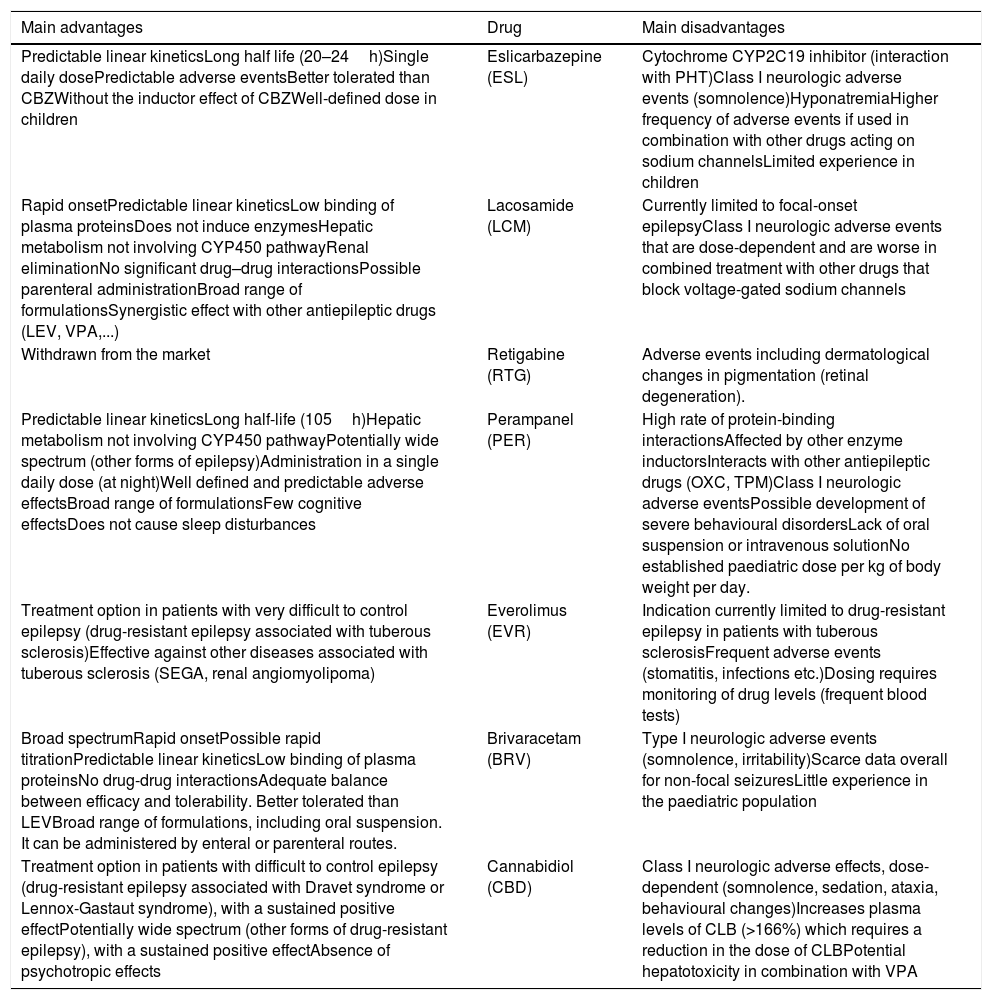
Main advantages and disadvantages of third-generation antiepileptic drugs in the paediatric population.
| Main advantages | Drug | Main disadvantages |
|---|---|---|
| Predictable linear kineticsLong half life (20–24h)Single daily dosePredictable adverse eventsBetter tolerated than CBZWithout the inductor effect of CBZWell-defined dose in children | Eslicarbazepine (ESL) | Cytochrome CYP2C19 inhibitor (interaction with PHT)Class I neurologic adverse events (somnolence)HyponatremiaHigher frequency of adverse events if used in combination with other drugs acting on sodium channelsLimited experience in children |
| Rapid onsetPredictable linear kineticsLow binding of plasma proteinsDoes not induce enzymesHepatic metabolism not involving CYP450 pathwayRenal eliminationNo significant drug–drug interactionsPossible parenteral administrationBroad range of formulationsSynergistic effect with other antiepileptic drugs (LEV, VPA,...) | Lacosamide (LCM) | Currently limited to focal-onset epilepsyClass I neurologic adverse events that are dose-dependent and are worse in combined treatment with other drugs that block voltage-gated sodium channels |
| Withdrawn from the market | Retigabine (RTG) | Adverse events including dermatological changes in pigmentation (retinal degeneration). |
| Predictable linear kineticsLong half-life (105h)Hepatic metabolism not involving CYP450 pathwayPotentially wide spectrum (other forms of epilepsy)Administration in a single daily dose (at night)Well defined and predictable adverse effectsBroad range of formulationsFew cognitive effectsDoes not cause sleep disturbances | Perampanel (PER) | High rate of protein-binding interactionsAffected by other enzyme inductorsInteracts with other antiepileptic drugs (OXC, TPM)Class I neurologic adverse eventsPossible development of severe behavioural disordersLack of oral suspension or intravenous solutionNo established paediatric dose per kg of body weight per day. |
| Treatment option in patients with very difficult to control epilepsy (drug-resistant epilepsy associated with tuberous sclerosis)Effective against other diseases associated with tuberous sclerosis (SEGA, renal angiomyolipoma) | Everolimus (EVR) | Indication currently limited to drug-resistant epilepsy in patients with tuberous sclerosisFrequent adverse events (stomatitis, infections etc.)Dosing requires monitoring of drug levels (frequent blood tests) |
| Broad spectrumRapid onsetPossible rapid titrationPredictable linear kineticsLow binding of plasma proteinsNo drug-drug interactionsAdequate balance between efficacy and tolerability. Better tolerated than LEVBroad range of formulations, including oral suspension. It can be administered by enteral or parenteral routes. | Brivaracetam (BRV) | Type I neurologic adverse events (somnolence, irritability)Scarce data overall for non-focal seizuresLittle experience in the paediatric population |
| Treatment option in patients with difficult to control epilepsy (drug-resistant epilepsy associated with Dravet syndrome or Lennox-Gastaut syndrome), with a sustained positive effectPotentially wide spectrum (other forms of drug-resistant epilepsy), with a sustained positive effectAbsence of psychotropic effects | Cannabidiol (CBD) | Class I neurologic adverse effects, dose-dependent (somnolence, sedation, ataxia, behavioural changes)Increases plasma levels of CLB (>166%) which requires a reduction in the dose of CLBPotential hepatotoxicity in combination with VPA |
In this article, we attempted to summarise the main characteristics of the new AEDs used in paediatrics. Most of these third-generation drugs are effective, safe and generally well tolerated in children. If we focused on those intended for broader use (BRV, ESL, LCM, PER), while their effectiveness has been inferred by the extrapolation of data from studies in the adult population, it will be the day-to-day clinical experience that will reveal the true potential of these drugs, probably leading to expanding their spectrum and defining new indications or synergistic effects with other treatments (as has been the case of most antiepileptic drugs in the past). A recently published, large meta-analysis (it analysed 19 double-blind randomised controlled trials, with n=7245) on the use of third-generation anticonvulsants (ESL, LCM, PER, BRV) in adults with focal epilepsy did not find significant differences in effectiveness, although the data suggested that BRV was better tolerated compared to the other drugs, independently of the dose used.36 Thus, taking into account that all of these drugs seem to be equally effective and safe, the management of epilepsy is becoming increasingly individualised, with selection of one drug or another based on tolerance, synergistic effects, interactions or contraindications, although the goal of treatment remains the same for all: to improve the quality of life of children with epilepsy and their families.
Please cite this article as: Málaga I, Sánchez-Carpintero R, Roldán S, Ramos-Lizana J, García-Peñas JJ. Nuevos fármacos antiepilépticos en Pediatría. An Pediatr (Barc). 2019;91:415.