Edited by: Fernando Santos-Simarro. Molecular Diagnostic and Clinical Genetics Unit University Hospital Son Espases. Palma de Mallorca. Spain
Last update: October 2025
More infoThe development of genomic technologies has transformed pediatric practice, enabling significant advances in the diagnosis of genetic diseases, 70% of which manifest during childhood. Genomic variants, ranging from single-nucleotide changes to large chromosomal rearrangements, are responsible for many pediatric conditions, and their detection relies on the appropriate selection of technologies. Methods such as karyotyping, MLPA, microarrays, Sanger sequencing, and next-generation sequencing (NGS) have increased diagnostic capacity, although, on average, a definitive diagnosis is currently made in only 27% of pediatric cases. Gene panels and exome, genome, and RNA sequencing offer varying diagnostic yields depending on clinical complexity, with rates that may be as high as 75% in specific cohorts. Additionally, emerging technologies such as long-read sequencing and optical genome mapping have proven useful in identifying complex structural variants and repetitive genomic regions. The integration of comprehensive clinical phenotyping and tools like the Human Phenotype Ontology (HPO) standard vocabulary optimizes genetic variant prioritization and enhances diagnostic accuracy. This article reviews the capabilities, limitations and clinical applications of currently available genomic techniques, highlighting their differences, advantages and disadvantages as well as implications for diagnostics in pediatrics.
El desarrollo de tecnologías genómicas ha transformado la práctica de la pediatría, permitiendo avances significativos en el diagnóstico de enfermedades genéticas, el 70% de las cuales debutan en la infancia. Las variaciones genómicas, que van desde cambios en un solo nucleótido hasta grandes reorganizaciones cromosómicas, son responsables de muchas enfermedades pediátricas, y su detección depende de la selección adecuada de tecnologías. Métodos como el cariotipo, MLPA, microarrays, secuenciación Sanger y secuenciación de nueva generación (Next Generation Sequencing; NGS) han incrementado la capacidad diagnóstica, aunque, en general, solo el 27% de los casos pediátricos alcanzan un diagnóstico definitivo. Los paneles de genes, exomas, genomas y RNAseq ofrecen diferentes rendimientos diagnósticos según la complejidad del caso clínico, con tasas que pueden alcanzar hasta el 75% en cohortes específicas. Además, tecnologías emergentes como la secuenciación de lectura larga y el mapeo óptico del genoma han demostrado utilidad en la identificación de variantes estructurales complejas y regiones repetitivas del genoma. La integración de un fenotipado clínico exhaustivo y herramientas como el vocabulario estandarizado de fenotipos de Human Phenotype Ontology (HPO) optimiza la priorización de variantes genéticas y mejora la precisión diagnóstica. Este artículo revisa las capacidades, limitaciones y aplicaciones clínicas de las técnicas genómicas disponibles en la actualidad, resaltando diferencias, ventajas y desventajas y sus implicaciones para el diagnóstico en pediatría.
Advances in genomic technologies have revolutionized the diagnosis of rare genetic diseases, 70% of which have onset in childhood.1 The development of efficient analytical tools has enabled the application of next-generation sequencing (NGS) and microarrays in clinical practice. Although these techniques have improved diagnostic yield, a definitive diagnosis is only achieved in 26.7% of pediatric cases.2
The diagnostic process can be long, complex and costly. Currently, the estimated average time for an accurate diagnosis is five years, while approximately 30% of children with a rare disease die before reaching five years of age.1 During this time, both patients and their families face significant emotional and financial challenges, while the health care system bears a substantial burden.
The analysis of genomic data has become an essential tool in today’s clinical practice. Having a genetic diagnosis contributes to:
- •
Understanding the cause and natural history of the disease and estimating life expectancy.
- •
Identifying other family members who may be at risk of developing the same condition and providing support in family planning through appropriate genetic counseling.
- •
Accessing specific treatments (if available for the disease) or participating in clinical trials for the development of a treatment.
- •
Being able to get in touch with other families in the same situation to share experiences and resources.
To maximize the usefulness of currently available molecular biology techniques, comprehensive and accurate patient phenotyping is essential, including detailed documentation of signs and symptoms and a thorough history-taking. Adequate phenotyping optimizes the analysis of genetic data, saving time and reducing the risk of ambiguous or inconclusive results.
Next generation sequencing (NGS) can detect thousands of genetic variants in a patient, many of which are variants of uncertain significance (VUS). However, a clearly defined phenotype allows prioritization of the variants that are most relevant to the clinical picture, excluding those that are less significant. Likewise, simultaneous analysis of the index case and the parents (trio analysis) frequently helps sidestep VUS by focusing on de novo variants or compound heterozygous variants inherited from both parents.
The Human Phenotype Ontology (HPO, https://hpo.jax.org/) is a tool that is widely used to standardize phenotypic data and investigate the association of phenotypes with genes.3 It allows the direct match of phenotypic data with genetic data, streamlining data analysis and the prioritization of relevant variants.
Types of genomic variation and their association with diseaseIn the field of genomics, variations in DNA are the basis of human diversity and, in many cases, the cause of disease. There are different types of variants, which can have different effects on health depending on their size and location in the genome. Structural variants (SVs) are large changes in the structure of chromosomes, such as deletions, duplications, inversions or translocations. They can affect large regions of the genome and, therefore, multiple genes. Copy number variants (CNVs) are a specific type of SV involving an increase or decrease in the number of copies of a particular region of the genome. Examples of diseases caused by copy number variation include Williams-Beuren syndrome, caused by a deletion on chromosome 7q11.23, cri du chat syndrome, caused by a deletion on the short arm of chromosome 5p15.2, or Prader-Willi and Angelman syndromes, caused by deletions on chromosome 15q11.2-q13. Single nucleotide variants (SNVs) are changes in a single nucleotide. Although these changes are small, they can have a major impact, especially if they occur in protein-coding regions or gene expression regulatory regions. Some examples of diseases commonly caused by this type of variation are cystic fibrosis, which is caused by mutations in the CFTR gene, sickle cell anemia, caused by a mutation in the hemoglobin gene, and phenylketonuria, caused by mutations in the PAH gene. Last of all, there is a third type of genomic variation known as short tandem repeats (STRs), short DNA sequences that are repeated consecutively a variable number of times and that can reach a pathological length. Most STR expansion disorders affect the nervous system. Some examples are fragile X syndrome, caused by the expansion of a CGG trinucleotide repeat in the FMR1 gene, or myotonic dystrophy, caused by expansion of a CTG trinucleotide repeat in the DMPK gene. In short, SVs, CNVs, SNVs and STR expansions are different types of genetic variation that may cause disease. The identification of variants is essential for diagnosis, genetic counseling and the development of novel therapies. In light of the above and the rapid pace of technological advancement, it is not uncommon for clinicians to experience uncertainty at the time of ordering genetic tests, pondering questions like “Which of the tests and techniques available now may offer the highest yield given the phenotype of the patient?” “What are the advantages and limitations of each technique?” or “What would be better, a test targeting a specific gene or syndrome or broad genetic testing covering the whole genome?”. From this point forward, we will attempt to answer these questions.
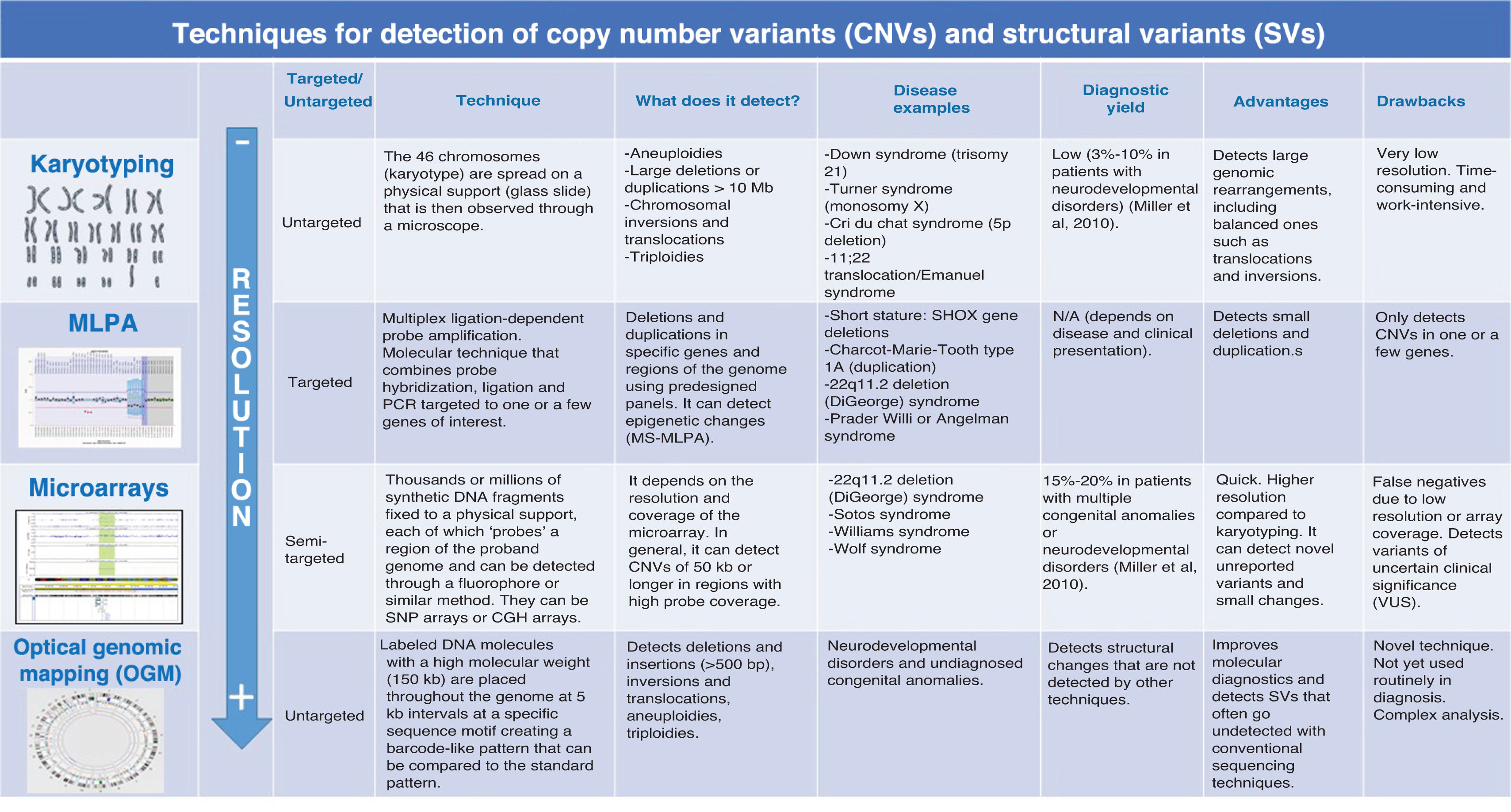
Comparison of karyotyping, MLPA and microarrays for detection of SVs and CNVsA comparison of karyotyping, multiplex ligation-dependent probe amplification (MLPA) and microarrays for detection of SVs in pediatric genetic disorders reveals that each technique has strengths and limitations that determine its utility based on the clinical and diagnostic context (Fig. 1).
Comparison of the different techniques that allow detection of CNVs and SVs.
Source: Miller et al., 2010.4
Karyotyping has been the main tool for analyzing chromosome number and structure for decades. It allows for the identification of major chromosomal abnormalities, such as aneuploidy (changes in chromosome number, as in Down syndrome, characterized by trisomy 21) and gross structural rearrangements, such as translocations, inversions or large deletions. However, its resolution is limited, as it can only detect abnormalities involving more than 5 to 10 Mb (million bases). Due to its low sensitivity, karyotyping is not useful for identifying microdeletions or microduplications, which are the cause of genomic syndromes involved in many pediatric genetic disorders. Nonetheless, it remains a fundamental technique in certain clinical situations, such as the diagnosis of aneuploidies or balanced translocations.
Multiplex ligation-dependent probe amplification constituted a significant advance in the capacity for detection of specific genetic variants. This technique uses probes designed for specific regions of the genome and allows identification of duplications or deletions in the genes of interest. There is a modality, methylation-specific MLPA (MS-MLPA) that can detect changes in DNA methylation associated with genomic imprinting disorders. Multiplex ligation-dependent probe amplification is particularly useful when specific genetic disorders are suspected, such as Duchenne muscular dystrophy, caused by changes in the DMD gene (MLPA), or Prader-Willi or Angelman syndrome (MS-MLPA). In contrast to its high sensitivity for these diseases, MLPA has the limitation of being unable to detect complex structural variations (such as inversions or translocations) or changes outside the specific regions covered by the probes. For this reason, its use is restricted to situations in which there is a clear and specific clinical suspicion.
In contrast, microarrays are a powerful tool for large-scale genomic analysis, allowing the detection of CNVs from approximately 50 kb. Their coverage is significantly greater compared to karyotyping or MLPA, making them especially useful in cases without a specific clinical suspicion. This technology facilitates the identification of microdeletions and microduplications throughout the genome, contributing to the diagnosis of genetic disorders that could go undetected with conventional methods. While most microarrays are not designed to detect point mutations or some complex structural variations, certain advanced formats (SNP arrays) can detect SNVs and regions with loss of heterozygosity. Still, their main application in diagnosis is for detection of submicroscopic chromosomal abnormalities, making microarrays a first-tier method in the investigation of genetic diseases. Some prominent examples are the detection of subtelomeric CNVs as a cause of idiopathic intellectual disability and duplications on chromosome 16p11.2, which are linked to autism.
Since each technique has its own applications and limitations, in real-world practice, several of these tools are frequently combined for a more comprehensive diagnosis depending on the patient’s phenotype and the suspected diseases.
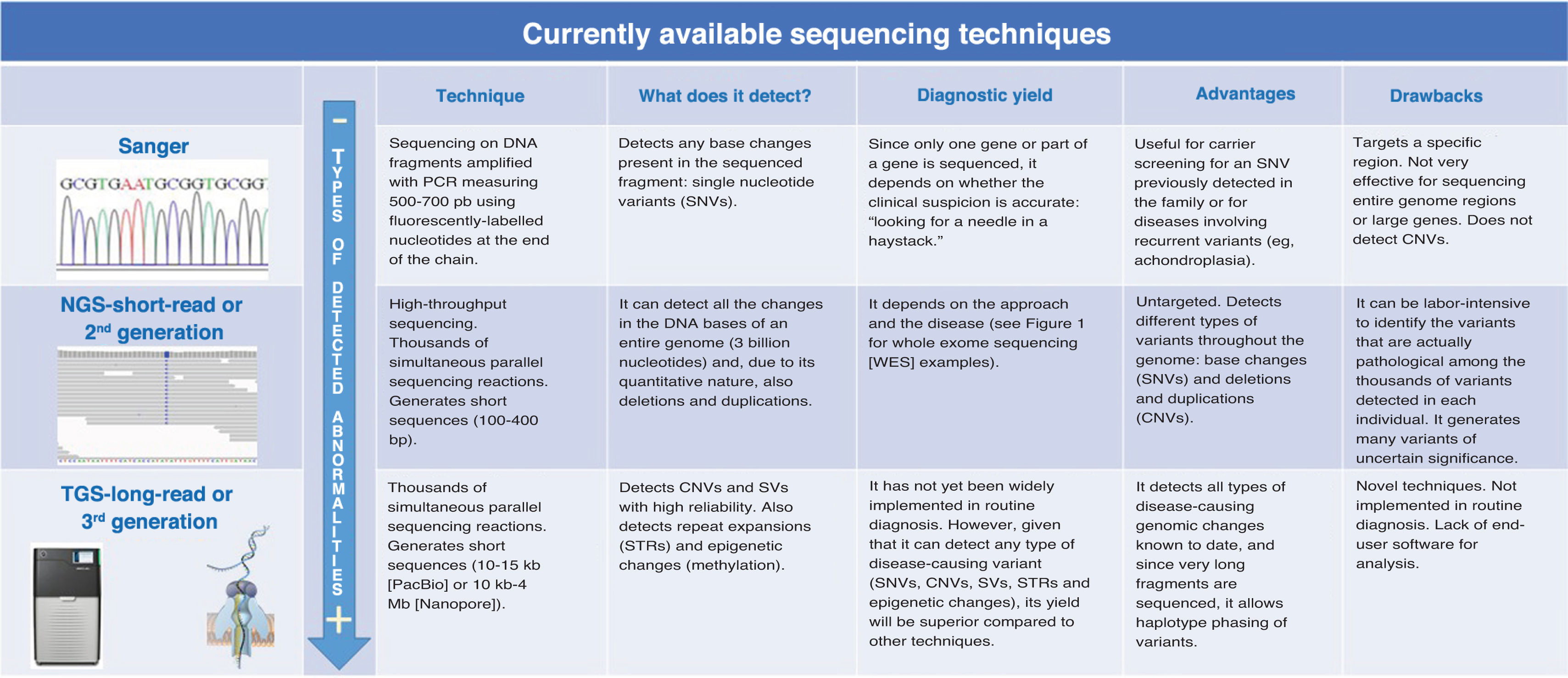
Comparison of Sanger and NGS sequencing and their application to the detection of SNVs and SVsSanger sequencing and NGS are both mainly used to analyze SNVs, but they have significant differences in terms of throughput, efficiency, cost and the types of variant they can detect (Fig. 2).
Sanger sequencing is primarily used to sequence small DNA fragments. Although it is an older technology, it continues to be widely used in clinical practice, especially for family studies of known variants that segregate with disease. It offers a high resolution for sequencing specific genes or exons, and, although it is limited to short sequences (approximately 600–800 bases), it has an extremely low error rate. Sanger sequencing is ideal for specific point mutations, such as those found in a single gene or a small region of the genome. It is a valid option for diagnostic confirmation when a known mutation in a specific gene is suspected.
Next generation sequencing, also known as massive parallel or second-generation sequencing, has revolutionized genetic diagnosis by enabling large-scale sequencing at a proportionally lower cost compared to conventional techniques. It allows simultaneous sequencing of large amounts of DNA. It can be used to sequence gene panels, whole exomes (exclusively the exons of genes) or whole genomes with a high resolution. It can detect SNVs, but also CNVs and SVs. Although NGS is more expensive than Sanger sequencing for analysis of a few genes, it is more efficient when large gene panels or the entire exome need to be analyzed. It can reduce the total time elapsed to diagnosis compared to conventional testing, especially when multiple genes are involved. In general, it has comparable accuracy to Sanger sequencing, although the error rates may be higher, especially in repetitive regions. Next generation sequencing is particularly useful in situations in which the phenotype is nonspecific or several genetic disorders are suspected. Some examples are autism, congenital heart disease or intellectual disability. The processing, analysis and interpretation of NGS data require advanced bioinformatics, which can be a barrier to its use. The detection of variants of uncertain significance can impede the establishment of a definitive diagnosis.
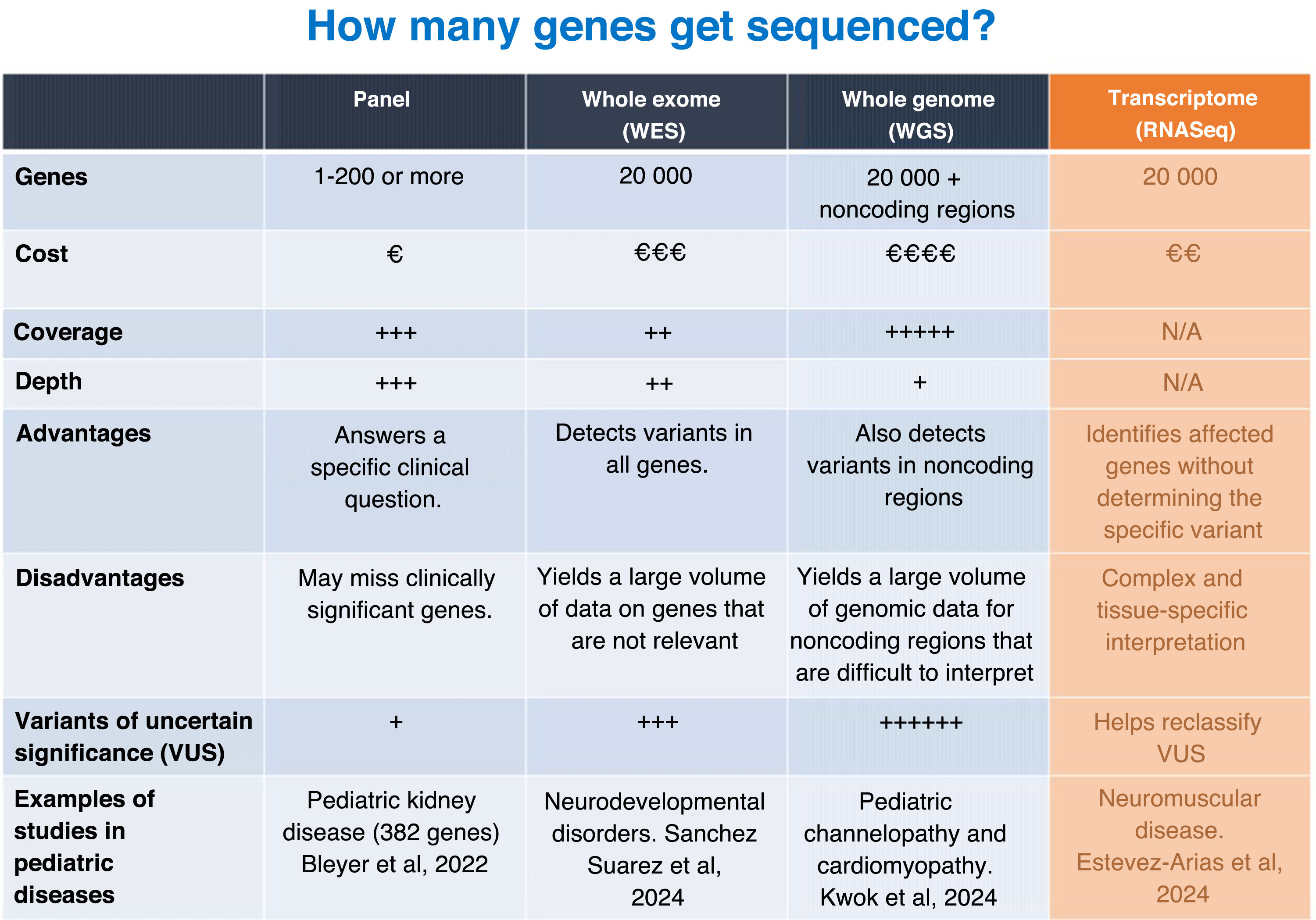
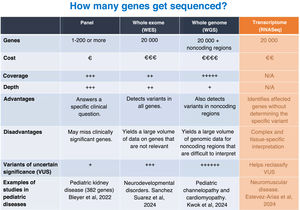
Utility of gene panels and exome, genome and RNA sequencingWithin NGS, the usefulness of gene panels and whole-exome, whole-genome and RNA sequencing for the diagnosis of pediatric diseases varies depending on the clinical context, budget and objectives of the analysis (Fig. 3).
Gene panels target limited sets of genes associated with specific diseases. They are generally designed for in-depth coverage of key genes associated with a particular phenotype. They are less costly than whole-exome or whole-genome sequencing and generate a smaller amount of data for analysis, facilitating the rapid identification of relevant variants. In addition, they minimize the probability of identifying genetic variants unrelated to the clinical presentation. Their main disadvantage is that they can fail in achieving a diagnosis when the phenotype is associated with genes other than those included in the panel. They are particularly useful for monogenic diseases that elicit a clear clinical suspicion (eg, muscular dystrophies, Rendu-Osler syndrome, Alport syndrome).
The exome is the part of the genome that contains the exons, which are the coding regions of the genes responsible for the production of proteins. In whole-exome sequencing (WES), approximately 1% to 2% of the genome is analyzed, but this small percentage accounts for up to 85% of the known disease-causing variants. One of the advantages of WES over gene panels is that it can detect variants in unexpected genes that can explain complex phenotypes. However, it cannot detect variants in regulatory regions or introns, which may also be clinically relevant. In addition, it identifies a large number of variants, which may increase the time required for its interpretation compared to a gene panel as well as the risk of identifying variants unrelated to the clinical presentation. Its use is recommended for complex phenotypes or when a specific set of genes is not suspected, although nowadays phenotype-driven virtual panels can be designed with bioinformatic methods to eliminate the genes that are not relevant for a given disease or phenotype, allowing the application of WES as if it were a collection of gene panels.
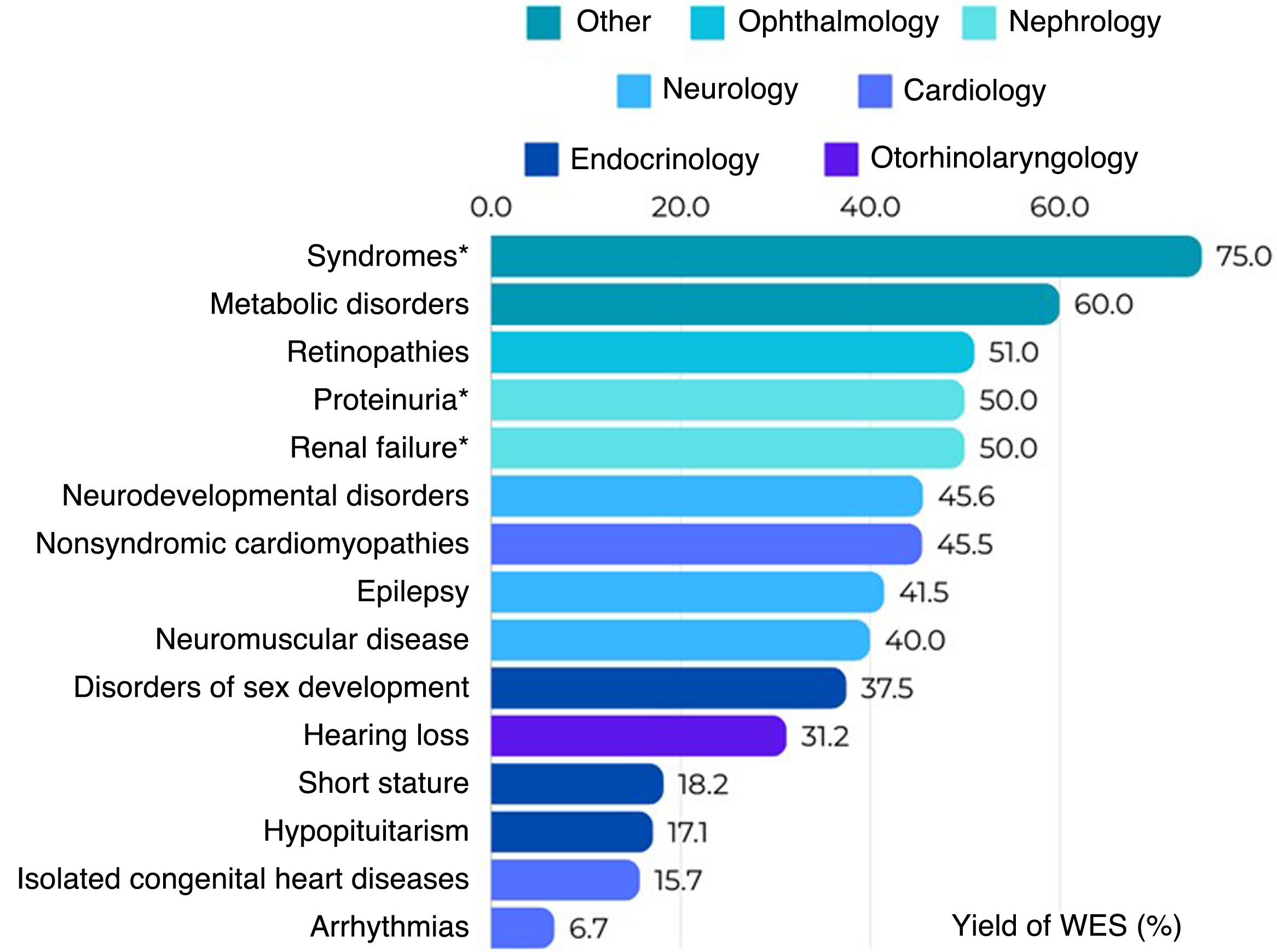
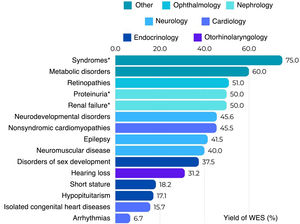
The reported diagnostic yield of WES applied to clinical practice ranges from 10% to more than 60%, depending on the clinical characteristics of the tested population, the year of testing and the analytical strategy.10Fig. 4 shows the diagnostic yield of WES calculated in different studies conducted in pediatric populations that were published recently (2023–2024). We ought to highlight is the high yield observed in patients with genetic syndromes (neurodevelopmental disorders plus other phenotypes such as facial dysmorphic features, epilepsy, congenital heart disease or dystonia) when trio WES is performed, which rises to 75%.11 In contrast, the lowest yields correspond to isolated arrhythmias and congenital heart diseases.12
Diagnostic yield of WES reported in different scientific publications by phenotype of interest.11–20 *Trio analysis.
Whole genome sequencing (WGS) involves sequencing the entire known DNA sequence. This tests covers most types of variation, as it detects variants in noncoding regions and introns and structural variants like inversions, duplications and deletions (CNVs). Analyzing the entire genome can eliminate the need for performing other specific tests to detect CNVs and SVs, such as microarrays, so WGS could be a first-tier test. However, to date, it has not yet been implemented in everyday practice in most laboratories and is reserved for diseases with a complex or unknown genetic etiology or cases in which variants have not been identified using other techniques or approaches.21 It is also suitable for investigating new genetic associations. It is more expensive than other approaches, although its cost has been decreasing. It also requires more time and resources for analysis and interpretation. Like WES, it can reveal unexpected or unwanted information (eg, predisposition to adult diseases).
Adding RNA sequencing (RNASeq) to the comparison between gene panels, WES and WGS provides a broader perspective, as RNASeq allows for the exploration of gene expression and events related to RNA processing (splicing). This technique consists in sequencing the transcriptome, which encompasses messenger RNA and other types of RNA. It detects changes in gene expression, splicing aberrations and fusion transcripts. It is useful in the diagnosis of diseases caused by variants that alter RNA expression or processing and identifies the functional effects of variants in noncoding regulatory regions or introns. It complements WES and WGS for the purpose of validating their findings. However, the results depend on the tissue used in testing, and, as a general rule, the RNA must come from the affected tissue to allow detection of relevant abnormalities. Furthermore, RNASeq does not detect genomic variants if they do not affect RNA. At present, it is rarely applied in routine diagnosis and its use is mostly restricted to research.
The choice of technique depends on the particular clinical case. A stepwise approach is common, starting with gene panels or WES, followed by WGS or RNASeq if a clear diagnosis cannot be established. In complex pediatric diseases, combining techniques (eg, WGS + RNASeq) may be key for maximizing diagnostic accuracy. In any case, both WGS and RNASeq are not yet used for routine diagnosis in Spain, and their application tends to be restricted to research.
Emerging genomic technologiesThird-generation sequencing (TGS), also known as long-read sequencing, and optical genome mapping (OGM) are advanced technologies that complement or may even replace tools such as NGS and microarrays in certain contexts. These techniques have proven particularly useful in the diagnosis of pediatric diseases, in which complex structural variants, difficult to detect by means of NGS, may be the cause of the disease.
Optical genome mapping allows detection of structural variants throughout the genome through the physical visualization of extremely long DNA molecules (up to hundreds of kilobases). Its high sensitivity makes it ideal for identifying translocations, inversions, large deletions, duplications, and copy number variants (CNVs), including those in repetitive DNA sequences and noncoding regions. Unlike NGS, it does not require amplification or sequencing, which reduces methodological biases. However, it has limitations in the detection of small variants (<500 bp) and its implementation remains costly in terms of equipment and analysis. It is useful for identifying structural alterations that are not detected by NGS, for instance, in some deletion and duplication syndromes or in certain cases of pediatric cancer.
On the other hand, long-read sequencing, or TGS, generates long DNA fragments (kilobases), allowing more accurate mapping of complex genomic regions. It is particularly useful for detecting structural variants that are difficult to resolve with the short reads of NGS and to analyze tandem repeats, which are relevant in diseases such as myotonic dystrophy or fragile X syndrome. Furthermore, when applied to RNASeq, it facilitates the identification of alternative RNA isoforms. Its advantages notwithstanding, TGS continues to be more expensive than NGS and has a higher initial error rate, requiring specialized infrastructure for both data generation and analysis.
Together, both OGM and TGS are significant advances that contribute to improving diagnosis in complex genetic cases, especially when standard testing (panels, WES, or WGS) has been inconclusive. Over time, technological advances and cost reductions will determine whether they can replace NGS as the first-tier method. However, the increase in yield associated with the use of these techniques remains limited, with improvements of no more than 10%, possibly due to nongenetic or polygenic factors.
ConclusionsNovel genomic technologies are revolutionizing pediatric diagnostics and improving the identification of genetic diseases, although challenges remain in the interpretation and accessibility of these tests. As technology advances, whole-exome and whole-genome sequencing are becoming increasingly relevant in clinical practice, facilitating faster and more accurate diagnoses. However, their implementation requires improvements in bioinformatics, data interpretation and accessibility. In this context, accurate phenotyping is key for optimizing genetic analysis and reducing uncertainty in the results. In addition, the combination of different techniques (karyotyping, MLPA, microarrays, NGS) allows for a more comprehensive evaluation of genomic variants.
The authors have no conflicts of interest to declare.