The aim of this study was to analyse the nutritional state, diet and gastrointestinal complications of children that require continuous renal replacement therapy (CRRT).
Material and methodsA retrospective analysis of a database, which included the information about patients who required CRRT between the years 2013 and 2017. Data were collected on the replacement technique, type of nutrition, calorie and protein intake, gastrointestinal complications, and clinical course. Children on extracorporeal membrane oxygenation (ECMO) were compared with the rest of patients.
ResultsA total of 65 children (61.5% male) were treated with CRRT, and 24 patients (37%) also needed ECMO support. Just over one-quarter (27.7%) of patients had a weight less than P3, and 48.4% of them a height less than P3. At the beginning of the technique, 31 children (47.7%) received enteral nutrition, at the end, there were 52 patients receiving enteral nutrition (80%). The transpyloric tube was used to provide nutrition in 76% of the cases. The median calorie intake was 63kcal/kg/day, and the protein intake was 1.6g/kg/day. There were gastrointestinal difficulties during the process in 48 patients (73.8%), with 29 (44.6%) patients being diagnosed with gastric distension or excessive gastric remains, 22 (33.8%) with constipation, 8 (12.3%) with vomiting, and 4 (6.1%) diarrhoea. One patient treated with ECMO presented with intestinal ischaemia. Enteral nutrition was cancelled in 3 patients (4.6%) due to the complications. There was no relationship between complications and type of diet or ECMO assistance.
ConclusionsA high percentage of children treated with CRRT showed undernutrition but they had adequate tolerance to the enteral nutrition. Although the gastrointestinal complications percentage was high in a the low number of subjects, these complications are the reason why enteral nutrition was stopped. ECMO patients do not show higher incidence of digestive complications.
El objetivo de este estudio ha sido analizar el estado de nutrición, la alimentación y las complicaciones digestivas de los niños que precisan técnicas de depuración extrarrenal continua (TDEC).
Material y métodosEstudio retrospectivo realizado sobre una base de datos prospectiva de los niños tratados con TDEC entre 2013 y 2017. Se analizaron las características de los pacientes, la técnica de depuración, el tipo de nutrición, el aporte calórico y proteico, las complicaciones digestivas y la evolución clínica.
Resultados65 niños (61,5% varones) fueron tratados con TDEC y 24 (37%) precisaron soporte con oxigenación con membrana extracorpórea (ECMO). Un 27,7% tenían un peso inferior al percentil 3 y un 48,4% una talla inferior al P3. Al inicio de la TDEC 31 niños (47,7%) recibían nutrición enteral y 52 (80%) al final de la misma. La nutrición enteral fue por sonda transpilórica en el 76%. La mediana de aporte calórico fue de 63kcal/kg/día y la del aporte proteico de 1,6g/kg/día. 48 pacientes (73,8%) presentaron complicaciones digestivas: 29 (44,6%) distensión gástrica o restos gástricos excesivos, 22 (33,8%) estreñimiento, 8 (12,3%) vómitos y 4 (6,1%) diarrea. Un paciente con ECMO presentó isquemia intestinal. En 3 pacientes (4,6%) se tuvo que suspender la nutrición enteral por complicaciones. No existió relación entre las complicaciones y el tipo de alimentación o la asistencia en ECMO.
ConclusionesUn elevado porcentaje de niños tratados con TDEC presentan malnutrición, pero la mayoría pueden ser alimentados con nutrición enteral. Aunque el porcentaje de complicaciones digestivas es elevado, en pocos pacientes se tiene que suspender la nutrición enteral.
A significant proportion of children admitted to paediatric intensive care units (PICUs) have acute kidney injury (AKI), and approximately 5% require renal replacement therapy.1,2 Continuous renal replacement therapy (CRRT) modalities are used most frequently, as they allow controlled fluid removal and unrestricted food intake.3,4
Children with AKI are at increased risk of undernutrition, which in turn is associated with increased morbidity and mortality.1–6 Furthermore, they often develop other complications, such as haemodynamic changes, need of mechanical ventilation and decreased gastric motility due to the administration of sedatives and muscle relaxants, which increase the risk of poor enteral feeding tolerance.1,2,4
On the other hand, replacement therapies may lead to loss of nutrients, amino acids, vitamins, folic acid or minerals like selenium through filtering.6
Few studies have analysed the characteristics of the nutrition given to children undergoing CRRT.4,6–8 One such study found a higher incidence of gastrointestinal complications in children with AKI compared to other critically ill children, and that this incidence increased the more severe renal impairment was in these patients.4
The aims of our study were to analyse the nutritional status, nutritional intake and gastrointestinal complications of children managed with CRRT.
Sample and methodsWe conducted a retrospective analysis of data collected prospectively in children admitted in the PICU and managed with CRRT between January 2013 and December 2017. The study was approved by the local ethics committee. We excluded patients treated with other forms of renal replacement therapy (peritoneal dialysis, immunoadsorption, plasma filtration).
We collected data on the following variables: demographic and clinical variables (age, sex, weight, height, body surface area, diagnosis); indication for and characteristics of CRRT, type of nutrition, mode of delivery of enteral nutrition (gastric tube feeding, transpyloric tube feeding, oral intake), type of diet, target intake (energy and protein), time elapsed until target intake was achieved with enteral nutrition, characteristics of parenteral nutrition (total volume, protein, carbohydrate and lipid intake in the first day), gastrointestinal complications (significant abdominal distension based on the judgment of the clinician in charge, gastric residual volume [50% of the volume administered in the past 4h], diarrhoea [more than 8 watery stools in infants aged up to 3 months, more than 4 loose stools in infants aged 3–12 months and more than 2 watery stools in children aged more than 12 months], constipation [>72h without a bowel movement despite administration of enteral nutrition], vomiting [vomiting at least twice in 24h]), bowel ischaemia (clinical signs [gastrointestinal bleeding, abdominal distension, decreased perfusion in abdominal wall] combined with sonographic or computed tomography findings compatible with bowel ischaemia), hypertransaminasaemia (alanine aminotransferase ALT>65IU/L and aspartate aminotransferase>400IU/L), need of extracorporeal membrane oxygenation (ECMO) and patient outcomes (survival, cause of death and length of stay in PICU).
Although nutritional management was customised to each patient, per the established protocol the initial intake targets were 60–65kcal/kg for energy and 1.5 a 2g/kg for protein, and in patients in who it was possible to calculate requirements using indirect calorimetry, the energy target was 1.3 times the resting energy expenditure.
We calculated weight and height percentiles and the corresponding z-scores using the nutrition application of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (Spanish Society of Paediatric Gastroenterology, Hepatology and Nutrition, https://www.seghnp.org/nutricional) using the charts published by Fernández et al. as reference, and we categorised nutritional status using the Waterlow indices for height (WIh) and weight (WIw), calculated with the following equations: WIh=[actual height/median (expected) height for age]×100; WIw=[actual weight/median (expected) weight for height]×100. We categorised nutritional status as9: normal WIw>90%, normal WIh>95%; acute undernutrition (mild, WIw=80–90%; moderate, WIw=70–80%; severe, WIw<70%), overnutrition (WIw>115%), chronic undernutrition (mild, WIh=90–95%, moderate, WIh=85–90%; severe, WIh<85%).
We analysed the association of feeding tolerance and gastrointestinal complications with the characteristics of the patient, nutritional support and the simultaneous use of ECMO and CRRT.
We performed the statistical analysis with the software IBM SPSS version 21.0. We summarised quantitative data as mean and standard deviation (SD) in case of a normal distribution and otherwise as median and interquartile range (IQR). We summarised qualitative data as percentages. To compare qualitative variables, we used the χ2 test and Fisher exact test. To compare the means of quantitative variables, we used the Student t test. We defined statistical significance as a p-value of less than 0.05.
ResultsWe analysed data for 65 children managed with CRRT over 5 years, of who 61.5% were male. The median age was 13.9 months (IQR, 4.6–80.7). The distribution of diagnoses at admission was: heart disease, 75%; abdominal surgery postoperative status, 5%, acute-on-chronic renal failure, 2%; sepsis, 3%; haemolytic uremic syndrome, 3%; respiratory failure, 3%; other, 9%. Of all patients, 92.3% were managed with mechanical ventilation and 36.9% with ECMO.
The median weight was 8.9kg (IQR, 5.6 to 18.7kg). The median weight z-score was −1.28 (IQR, −0.78 to −1.93). Eighteen patients (27.7%) had a body weight below the 3rd percentile, and 31 (48.4%) a height below the 3rd percentile. We found acute undernutrition (WIw<90%) in 32.8% of the sample (mild in 61.9% of cases, moderate in 19% and severe in 19%) and chronic malnutrition (WIh<95%) in 60.9% (mild in 38.5% of cases, moderate in 33.3% and severe in 28.2%).
Continuous renal replacement therapy was initiated at a median of 3 days after admission to the PICU (IQR, 1–7 days). In all cases, it consisted of continuous venovenous haemofiltration with a Prismaflex® system (Baxter). The median duration of CRRT was 7 days (IQR, 4–16 days). The reason for discontinuation of CRRT was recovery of renal function in 70.8% of patients, death in 24.6% and switch to a different renal replacement therapy modality in 4.6%.
At initiation of CRRT, 81.5% of patients were receiving nutritional support (47.7% enteral support and 33.8% parenteral support) and 18.5% were under nil per os with fluid replacement therapy.
In the group of patients managed with enteral nutrition, the mode of delivery was transpyloric tube feeding in 75.5%, gastric tube feeding in 20.8% and oral nutrition in 3.8%.
The foods administered through enteral nutrition were: infant formula in 6%, human milk in 9%, hypercaloric infant formula (Infatrini®) in 21%, protein hydrolysate formula in 19%, hypercaloric child formula (Isosource® Junior) in 30%, a formula high in medium chain triglycerides (Monogen®) in 9%, and a formula specific for individuals with kidney injury (Suplena®) in 6%. The median time elapsed to achieving the target intake in the enteral nutrition group was 6h (IQR, 3−12h). The median target enteral feeding volume was of 64mL/kg/day (IQR, 36−100mL), with an energy intake of de 63kcal/kg/day (IQR, 43−72kcal) and a protein intake of 1.6g/kg/day (IQR, 0.97–2.2g). We did not find significant differences in the energy intake or protein intake between children managed with gastric tube feeding (median of 60kcal/kg and 1.62g of protein/kg) and those managed with transpyloric tube feeding (median of 67kcal/kg and 1.55g of protein/kg).
The median parenteral nutrition volume delivered on day 1 was 50mL/kg/day (IQR, 39.4–62.9mL), with delivery of 53.3kcal/kg/day (IQR, 49–66.7kcal), 2.0g of protein/kg/day (IQR, 1.5–2.9g), 8.2g of carbohydrates/kg/day (IQR, 7,7–10,2g) and 1.1g/kg/day of fluids (IQR, 1–2g).
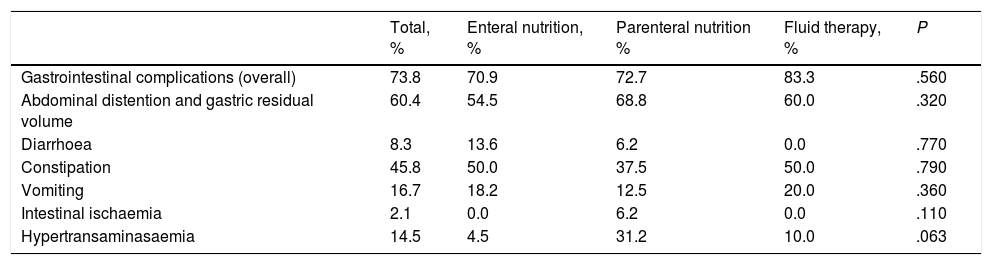
Gastrointestinal complications developed in 48 patients (73.8%) during CRRT. Table 1 presents the gastrointestinal complications observed in our patients by type of nutritional support. We did not find differences in the frequency of abdominal distension, presence of gastric residual volumes, vomiting, diarrhoea or constipation between patients initially managed with enteral nutrition, parenteral nutrition or fluid replacement therapy. Only 1 patient (2.1%), who was managed with parenteral nutrition, developed intestinal ischaemia. Hypertransaminasaemia was more frequent in the parenteral nutrition group, although the difference was not statistically significant. Enteral nutrition had to be discontinued in 3 patients due to suspected intestinal ischaemia, paralytic ileus and rhabdomyosarcoma of the biliary tree, and had to be reduced in 4 patients with addition of parenteral nutrition (due to intramural haematoma of the duodenum or abdominal distension). We found no differences in the incidence of gastrointestinal complications between children managed with gastric tube feeding (abdominal distension, 40%; diarrhoea, 10%; constipation, 50%) and children managed with transpyloric tube feeding (abdominal distension, 48.2%; diarrhoea, 6.8%; constipation, 51.7%), except in the incidence of vomiting, which was higher in the gastric tube group compared to the transpyloric tube group (40% vs 6.8%; P=.01).
Frequency of gastrointestinal complications. Comparison by type of nutrition.
| Total, % | Enteral nutrition, % | Parenteral nutrition % | Fluid therapy, % | P | |
|---|---|---|---|---|---|
| Gastrointestinal complications (overall) | 73.8 | 70.9 | 72.7 | 83.3 | .560 |
| Abdominal distention and gastric residual volume | 60.4 | 54.5 | 68.8 | 60.0 | .320 |
| Diarrhoea | 8.3 | 13.6 | 6.2 | 0.0 | .770 |
| Constipation | 45.8 | 50.0 | 37.5 | 50.0 | .790 |
| Vomiting | 16.7 | 18.2 | 12.5 | 20.0 | .360 |
| Intestinal ischaemia | 2.1 | 0.0 | 6.2 | 0.0 | .110 |
| Hypertransaminasaemia | 14.5 | 4.5 | 31.2 | 10.0 | .063 |
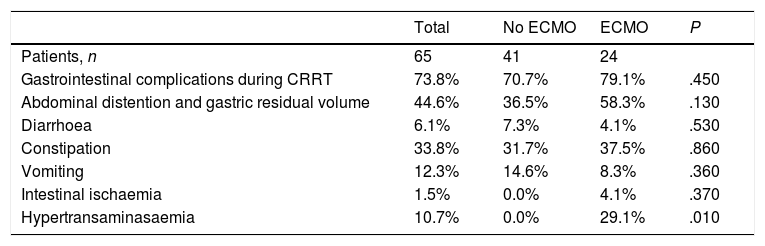
Twenty-four children (36.9%) were treated simultaneously with ECMO and CRRT. Gastrointestinal complications developed in 79.1% of these patients compared to 70.7% of patients treated with CRRT alone (P=.45). Table 2 compares the gastrointestinal complications found in patients managed with ECMO and without ECMO.
Gastrointestinal complications in children managed with ECMO versus children managed without ECMO.
| Total | No ECMO | ECMO | P | |
|---|---|---|---|---|
| Patients, n | 65 | 41 | 24 | |
| Gastrointestinal complications during CRRT | 73.8% | 70.7% | 79.1% | .450 |
| Abdominal distention and gastric residual volume | 44.6% | 36.5% | 58.3% | .130 |
| Diarrhoea | 6.1% | 7.3% | 4.1% | .530 |
| Constipation | 33.8% | 31.7% | 37.5% | .860 |
| Vomiting | 12.3% | 14.6% | 8.3% | .360 |
| Intestinal ischaemia | 1.5% | 0.0% | 4.1% | .370 |
| Hypertransaminasaemia | 10.7% | 0.0% | 29.1% | .010 |
CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation.
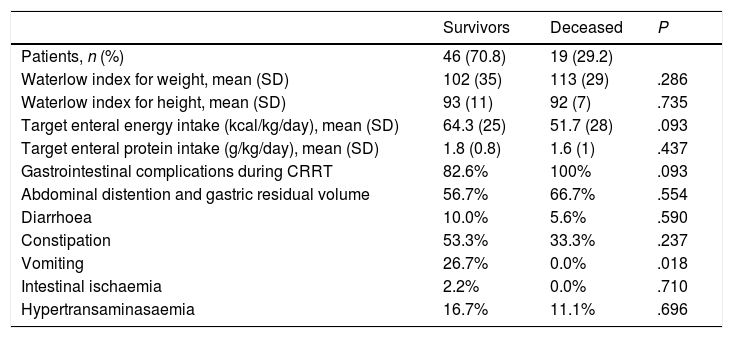
The median length of stay in the PICU was of 34.5 days (IQR, 21–58.2 days). Nineteen patients died (29.2%). The most frequent cause of death was multiple organ failure (57.9%), followed by cardiac complications (21.1%), brain death (5.3%) and intestinal ischaemia (5.3%). We found body weights below the 3rd percentile in 28.9% of survivors and 21.1% of patients that died (P=.758); 23.5% of children with weights below the 3rd percentile died compared to 31.9% of children with weights greater than the 3rd percentile (P=.758). Furthermore, 46.5% of survivors and 50% of deceased patients had heights below the 3rd percentile (P=.810). We did not find differences in the WIw or the WIh between survivors and the deceased (Table 3).
Comparison of nutritional status, nutritional intake and gastrointestinal complications between survivors and deceased patients.
| Survivors | Deceased | P | |
|---|---|---|---|
| Patients, n (%) | 46 (70.8) | 19 (29.2) | |
| Waterlow index for weight, mean (SD) | 102 (35) | 113 (29) | .286 |
| Waterlow index for height, mean (SD) | 93 (11) | 92 (7) | .735 |
| Target enteral energy intake (kcal/kg/day), mean (SD) | 64.3 (25) | 51.7 (28) | .093 |
| Target enteral protein intake (g/kg/day), mean (SD) | 1.8 (0.8) | 1.6 (1) | .437 |
| Gastrointestinal complications during CRRT | 82.6% | 100% | .093 |
| Abdominal distention and gastric residual volume | 56.7% | 66.7% | .554 |
| Diarrhoea | 10.0% | 5.6% | .590 |
| Constipation | 53.3% | 33.3% | .237 |
| Vomiting | 26.7% | 0.0% | .018 |
| Intestinal ischaemia | 2.2% | 0.0% | .710 |
| Hypertransaminasaemia | 16.7% | 11.1% | .696 |
CRRT, continuous renal replacement therapy SD, standard deviation.
The initial management in the PICU of 54.3% of survivors and 31.6% of deceased patients included enteral nutrition (P=10).
All patients that died experienced some form of gastrointestinal complication, but no specific gastrointestinal complication was significantly more frequent in deceased patients compared to survivors. On the contrary, vomiting was more frequent in survivors compared to deceased patients.
DiscussionAlthough several studies have analysed the association between malnutrition and AKI and enteral nutrition tolerance in adults and children managed with CRRT,1–7 our study is the first to analyse the nutritional status of these children, the characteristics of nutritional support, treatment with ECMO and CRRT and mortality.
A substantial proportion of children admitted to the PICU are malnourished, and malnutrition is associated with poorer outcomes.9,10 Kyle et al. found that a higher proportion of children with AKI not managed with CRRT had acute malnutrition (33%) compared to children without AKI.2 Another previous study that analysed the nutritional status of 174 children managed with CRRT found that 35% had body weights below the 3rd percentile and 56% had a weight-for-height of less than 0.85.11 The mortality of children with a body weight below the 3rd percentile was significantly higher compared to all other patients (51% versus 33%; P=.037). The multivariate analysis found that underweight was significantly associated with mortality.11 Our study also revealed that a high proportion of children managed with CRRT had acute or chronic malnutrition, but we did not find evidence of an association between nutritional status and mortality.
The energy requirements of critically ill children in the first days in the PICU range from 40 to 60kcal/kg/day.12 No studies in the literature have analysed the energy intake of children with AKI undergoing CRRT. Although indirect calorimetry is the ideal method to estimate the energy requirements of patients,8 this technique is available in few facilities. In our study, nutritional management achieved an energy intake of 63kcal/kg/day, but we were unable to determine whether this intake was sufficient.
Achieving an adequate protein intake is an essential goal of nutritional management in critically ill children, as it is necessary to prevent muscle catabolism and promote protein synthesis.13 A protein intake of at least 1.5g/kg/day is recommended for critically ill children.12 A decreased protein intake is generally recommended in children with AKI, but in children treated with CRRT, this technique carries out the filtering function of the kidney and thus it is not necessary to restrict protein intake. Some authors consider that children with AKI managed with CRRT require a protein intake of 1.5 to 2.5g/kg/day to compensate losses to filtration and guarantee a positive nitrogen balance.8,14
In our sample, children managed with enteral nutrition received an amount of protein at the lower limit of the recommended range (1.6g/kg/day), although we did not analyse the nitrogen balance. In opposition, protein intake was higher on day 1 of nutritional support in children managed with parenteral nutrition (2g/kg/day). These findings were consistent with those of a study conducted by Wong Vega et al. in 41 children managed with CRRT, in which those receiving enteral nutrition had a lower protein intake compared to those managed with parenteral nutrition.7
Enteral nutrition is the nutritional support modality of choice in most critically ill children because it preserves normal bowel physiology, stimulates the immune system, reduces bacterial translocation and is associated with a decreased incidence of sepsis and multiple organ failure.1 Parenteral nutrition should be reserved for patients that cannot tolerate enteral nutrition or when the latter cannot deliver sufficient amounts of energy and protein.13 However, there is a widespread belief that AKI can reduce tolerance of enteral nutrition, resulting in the management of many critical patients with AKI with parenteral nutrition.2,4,7 A recent study found that only 12% of children managed with CRRT received exclusive enteral nutrition.7 However, in our experience transpyloric tube feeding is a good alternative in critically ill children, as it prevents the problems associated with inadequate gastric emptying, can be used in patients under deep sedation and muscle relaxation, and allows a quick increase of feeding volumes to meet the target energy intake.15–17 In our study, transpyloric tube feeding was the modality used in 75% of patients managed with enteral nutrition and was associated with a decreased frequency of vomiting compared with gastric tube feeding. This could explain the higher proportion of patients that received enteral nutrition compared to other studies. For this reason, we believe that transpyloric tube feeding could be considered the first line of nutritional support in critically ill children under CRRT.4
Very few studies have analysed the tolerability and adverse effects of enteral nutrition in patients with AKI.4 We found a high incidence of gastrointestinal complications in our patients, in agreement with the findings of a previous study in which the frequency of complications was significantly higher in children with AKI compared to all other critically ill children.4
The most frequent gastrointestinal complication in our sample was abdominal distension, followed by constipation, vomiting and diarrhoea, with no significant differences between children managed with enteral versus parenteral nutrition. This suggests that these complications are not a direct result of nutrition and rather depend on the disease of the patient and its severity.
In our study, enteral nutrition only had to be permanently discontinued in 3 patients, and this was not a direct consequence of nutritional support in any case. Our findings show that while the incidence of gastrointestinal complications in children managed with enteral nutrition is high, most of them are not serious and do not require definitive discontinuation of enteral nutrition.
There is a dearth of data on the nutritional management of children treated with ECMO. Some authors have reported the nearly exclusive use of parenteral nutrition due to the potential risk of inadequate intestinal perfusion and mesenteric ischaemia giving rise to necrotising enterocolitis, intestinal ischaemia, intestinal perforation or gastrointestinal bleeding, while others have reported the use of enteral nutrition.18–20
A substantial percentage of patients in the sample (36.9%) were managed with ECMO and CRRT at the same time. We have not found any previous study analysing nutrition in critical patients managed concurrently with both techniques. We found a slightly higher incidence of gastrointestinal complications in children in this group compared to children managed with CRRT alone, but the difference was not statistically significant. Our results suggest that enteral nutrition is safe in children managed with both ECMO and CRRT and should be the first-line nutritional therapy in these patients once respiratory and haemodynamic stability is achieved.
On the other hand, there are no published studies analysing the association of nutritional status, type of nutritional support, energy intake and protein intake with patient outcomes in children with AKI managed with CRRT. Our study did not find evidence of an association between the type of nutritional management (enteral or parenteral) and mortality. We did not find a higher incidence of gastrointestinal complications in patients that died compared to survivors. Only 1 patient died from a gastrointestinal complication (intestinal ischaemia), and the complication was not secondary to nutrition. These findings support the use of enteral nutrition as the first line of nutritional management in these patients.
There are several limitations to our study. It was a retrospective study with a relatively small sample of patients and in a single PICU, and therefore multicentre studies are required to confirm our findings. In addition, the energy requirements of patients were not determined by indirect calorimetry and we did not analyse the nitrogen balance. Thus, we do not know whether the energy and protein intakes were adequate in each patient. We only compared the energy and protein intake in the group managed with enteral nutrition versus the group managed with parenteral nutrition on day 1, as the highest amounts delivered were not documented. Furthermore, we did not assess the potential impact of other factors, such as disease severity, the patient’s diagnosis and the use of other drugs on enteral tolerance and the development of gastrointestinal complications.
In conclusion, there is a high incidence of undernutrition in critically ill children with AKI requiring CRRT, but undernutrition is not associated with an increase in mortality. Most children treated with CRRT tolerate enteral nutrition, and while there is a high probability that they will experience gastrointestinal complications, these seldom require discontinuation of enteral nutrition. Children treated with ECMO and CRRT simultaneously did not exhibit a higher incidence of gastrointestinal complications compared to children treated with CRRT alone.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Lozano MJS, Álvarez CA, Heidbüchel CA, Lafever SF, García MJS, Cid JL-H. Nutrición en niños tratados con técnicas de depuración extrarrenal continua. An Pediatr (Barc). 2020;92:208–214.