A progressive increase in the incidence of infections caused by multidrug-resistant microorganisms is being reported. Among these resistant microorganisms, the main threats are extended-spectrum β-lactamase-, AmpC-, and carbapenemase-producing Gram-negative bacilli, methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. To address this important problem, it is essential to establish pediatric Antimicrobial Stewardship programs, perform active epidemiological surveillance and develop an adequate infection control policy. The therapeutic approach of these infections is often complex, frequently requiring antibiotics with less experience in children. In this position document made by the Spanish Association of Pediatrics and the Spanish Society of Pediatric Infectious Diseases, the epidemiology and treatment of these infections are reviewed according to the best available evidence.
En los últimos años se ha evidenciado un incremento en la incidencia de infecciones por bacterias multirresistentes. Las principales amenazas son los bacilos gramnegativos productores de β-lactamasas de espectro extendido (BLEE), AmpC o carbapenemasas, Staphylococcus aureus resistente a meticilina y Enterococcus faecium resistente a vancomicina. Para hacer frente a este problema, es fundamental establecer programas de optimización en el uso de antimicrobianos (PROA) específicos para pediatría, realizar una vigilancia epidemiológica activa y desarrollar una adecuada política de control de infecciones. Su abordaje terapéutico es a menudo complejo y multidisciplinar, precisando frecuentemente el uso de antibióticos con menor experiencia. En este documento de posicionamiento, elaborado por la Asociación Española de Pediatría y la Sociedad Española de Infectología Pediátrica, se revisa la epidemiologia y el tratamiento de estas infecciones siguiendo la mejor evidencia disponible.
Antimicrobial resistance is currently one of the main problems in public health.1 The prevalence of multidrug-resistant (MDR) bacteria has increased significantly in recent decades, and the World Health Organization (WHO) has included antimicrobial resistance in its list of 10 global threats to public health.
Infections by MDR organisms are particularly important in health care settings. However, they have also increased in frequency in community settings.2 These infections have a poorer prognosis due to the delay in initiation of appropriate antibiotic therapy and the need for alternative antibiotics, which are usually less effective and are less safe.3,4
The risk factors for infection by MDR bacteria are previous colonization, prior broad-spectrum antibiotic therapy, prolonged hospitalization, admission to intensive care, immunosuppression or the use of invasive medical devices.4,5 Measures for infection control in inpatients (such as hand hygiene, isolation of patients colonised by MDR bacteria) and antimicrobial stewardship programmes are essential strategies in addressing this emergent public health problem.
The treatment of infections by MDR bacteria may also involve the use of antimicrobials for which there is less information, some have been used for decades (e.g. colistin or fosfomycin), while others have been authorised recently6 (e.g. tigecycline, ceftolozane-tazobactam or ceftazidime-avibactam), with little evidence available regarding their use in paediatric population. Most of the current evidence comes from observational studies conducted in adults,7,8 and clinicians frequently need to resort to the off-label use of the few available antimicrobials.
Treatment must be individualised taking into account the severity of the disease, the characteristics of the patient, the source of the infection and the antimicrobial susceptibility profile of the isolated bacteria. In addition to antimicrobial treatment, it is also essential to provide supportive care and source control of the infection, whenever possible. Generally, we recommend consultation with experts any time a MDR strain is isolated from a clinical sample. Tables 1 and 2 list the doses of the antibiotics used most frequently for treatment of MDR bacterial infections. The duration of antibiotherapy should be determined based on the best available evidence.9
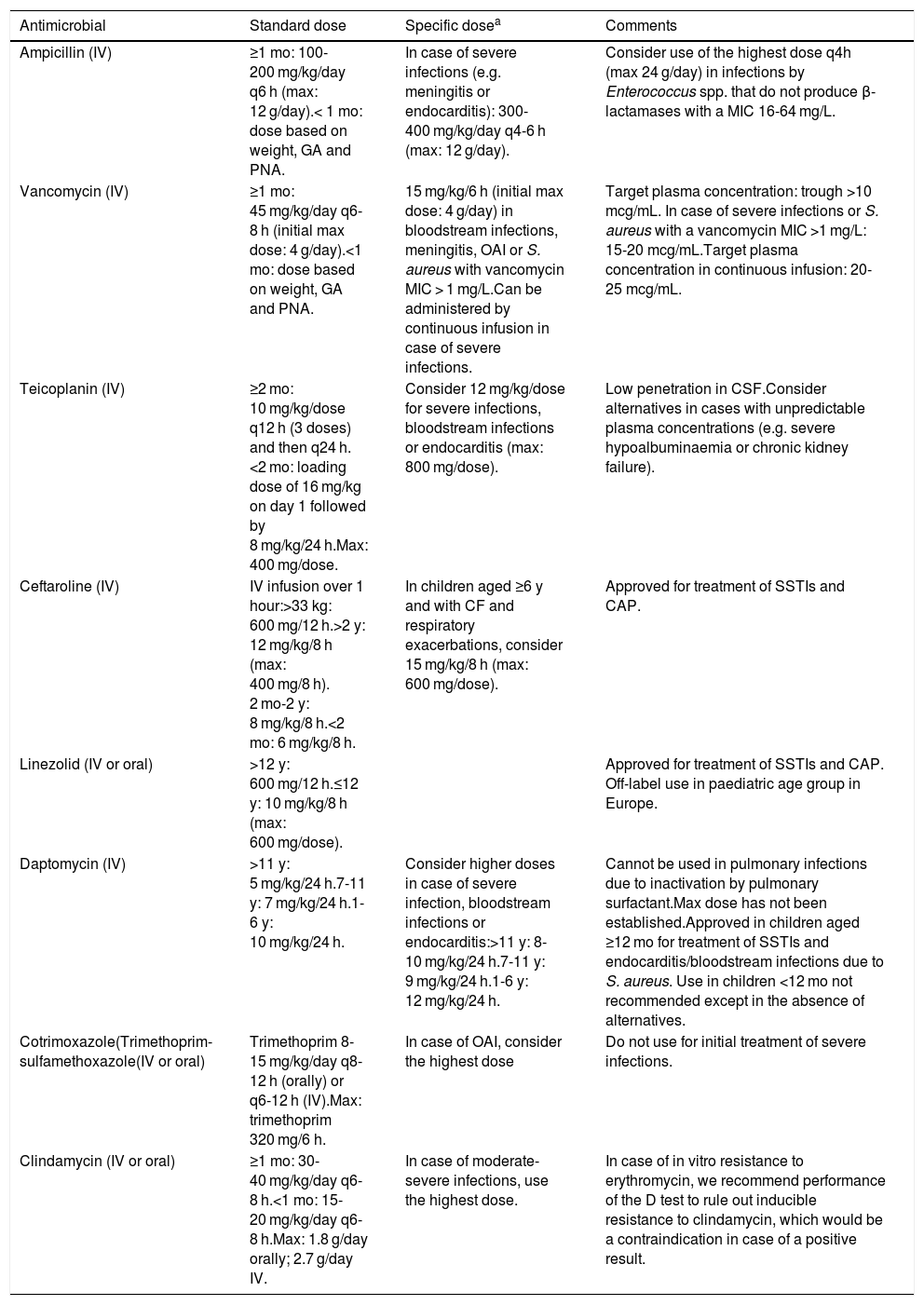
Dosage of the main antimicrobial agents used in paediatrics for treatment of infection by multidrug-resistant Gram-positive cocci.
| Antimicrobial | Standard dose | Specific dosea | Comments |
|---|---|---|---|
| Ampicillin (IV) | ≥1 mo: 100-200 mg/kg/day q6 h (max: 12 g/day).< 1 mo: dose based on weight, GA and PNA. | In case of severe infections (e.g. meningitis or endocarditis): 300-400 mg/kg/day q4-6 h (max: 12 g/day). | Consider use of the highest dose q4h (max 24 g/day) in infections by Enterococcus spp. that do not produce β-lactamases with a MIC 16-64 mg/L. |
| Vancomycin (IV) | ≥1 mo: 45 mg/kg/day q6-8 h (initial max dose: 4 g/day).<1 mo: dose based on weight, GA and PNA. | 15 mg/kg/6 h (initial max dose: 4 g/day) in bloodstream infections, meningitis, OAI or S. aureus with vancomycin MIC > 1 mg/L.Can be administered by continuous infusion in case of severe infections. | Target plasma concentration: trough >10 mcg/mL. In case of severe infections or S. aureus with a vancomycin MIC >1 mg/L: 15-20 mcg/mL.Target plasma concentration in continuous infusion: 20-25 mcg/mL. |
| Teicoplanin (IV) | ≥2 mo: 10 mg/kg/dose q12 h (3 doses) and then q24 h. <2 mo: loading dose of 16 mg/kg on day 1 followed by 8 mg/kg/24 h.Max: 400 mg/dose. | Consider 12 mg/kg/dose for severe infections, bloodstream infections or endocarditis (max: 800 mg/dose). | Low penetration in CSF.Consider alternatives in cases with unpredictable plasma concentrations (e.g. severe hypoalbuminaemia or chronic kidney failure). |
| Ceftaroline (IV) | IV infusion over 1 hour:>33 kg: 600 mg/12 h.>2 y: 12 mg/kg/8 h (max: 400 mg/8 h). 2 mo-2 y: 8 mg/kg/8 h.<2 mo: 6 mg/kg/8 h. | In children aged ≥6 y and with CF and respiratory exacerbations, consider 15 mg/kg/8 h (max: 600 mg/dose). | Approved for treatment of SSTIs and CAP. |
| Linezolid (IV or oral) | >12 y: 600 mg/12 h.≤12 y: 10 mg/kg/8 h (max: 600 mg/dose). | Approved for treatment of SSTIs and CAP. Off-label use in paediatric age group in Europe. | |
| Daptomycin (IV) | >11 y: 5 mg/kg/24 h.7-11 y: 7 mg/kg/24 h.1-6 y: 10 mg/kg/24 h. | Consider higher doses in case of severe infection, bloodstream infections or endocarditis:>11 y: 8-10 mg/kg/24 h.7-11 y: 9 mg/kg/24 h.1-6 y: 12 mg/kg/24 h. | Cannot be used in pulmonary infections due to inactivation by pulmonary surfactant.Max dose has not been established.Approved in children aged ≥12 mo for treatment of SSTIs and endocarditis/bloodstream infections due to S. aureus. Use in children <12 mo not recommended except in the absence of alternatives. |
| Cotrimoxazole(Trimethoprim-sulfamethoxazole(IV or oral) | Trimethoprim 8-15 mg/kg/day q8-12 h (orally) or q6-12 h (IV).Max: trimethoprim 320 mg/6 h. | In case of OAI, consider the highest dose | Do not use for initial treatment of severe infections. |
| Clindamycin (IV or oral) | ≥1 mo: 30-40 mg/kg/day q6-8 h.<1 mo: 15-20 mg/kg/day q6-8 h.Max: 1.8 g/day orally; 2.7 g/day IV. | In case of moderate-severe infections, use the highest dose. | In case of in vitro resistance to erythromycin, we recommend performance of the D test to rule out inducible resistance to clindamycin, which would be a contraindication in case of a positive result. |
CF, cystic fibrosis; CR, carbapenem-resistant; CSF, cerebrospinal fluid; EMA, European Medicines Agency; GA, gestational age; IV, intravenous; MIC, minimum inhibitory concentration; OAI, osteoarticular infection; PNA, postnatal age.
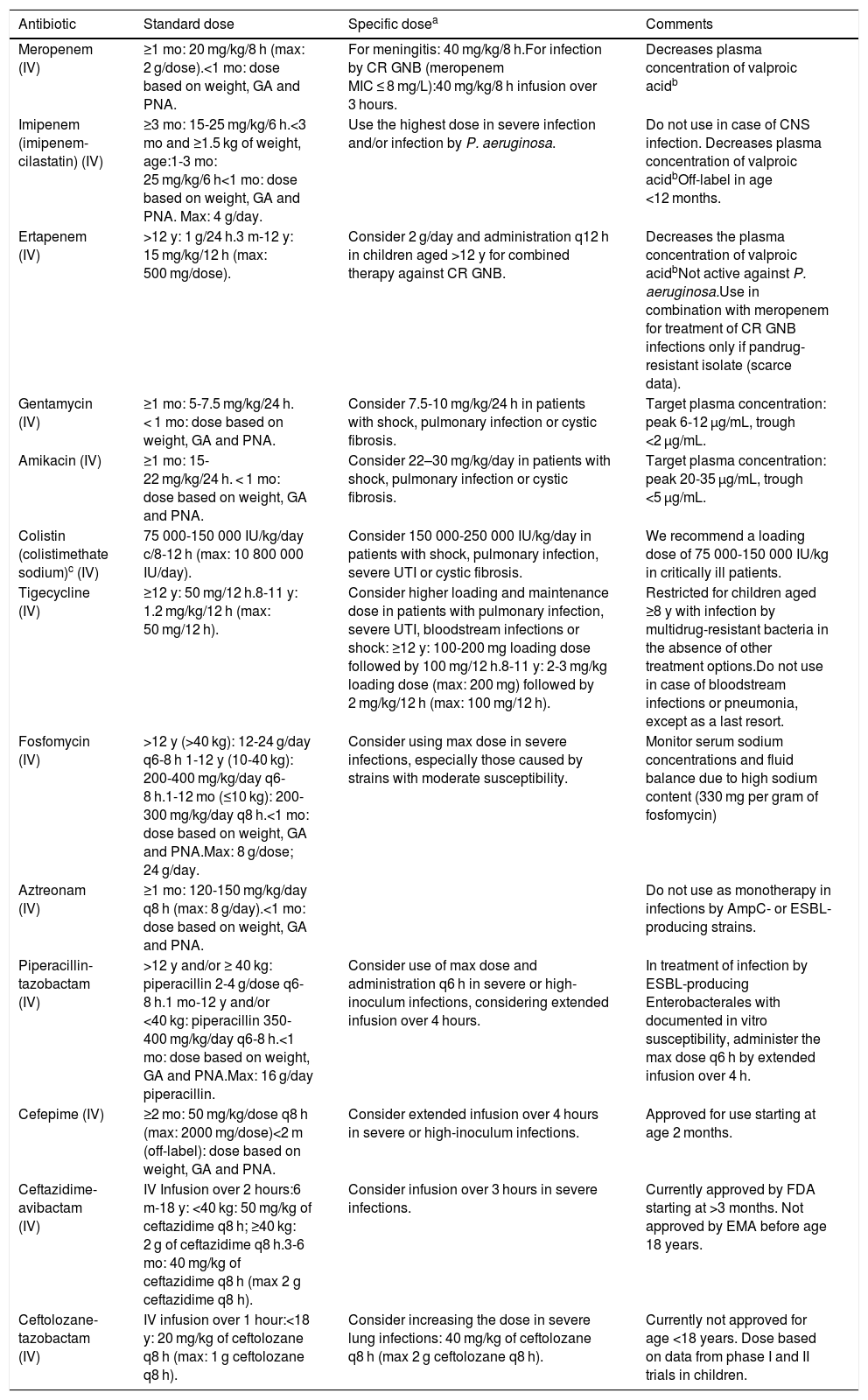
Dose of the main antimicrobials used in paediatrics for treatment of infections by drug-resistant Gram-negative bacilli.
| Antibiotic | Standard dose | Specific dosea | Comments |
|---|---|---|---|
| Meropenem (IV) | ≥1 mo: 20 mg/kg/8 h (max: 2 g/dose).<1 mo: dose based on weight, GA and PNA. | For meningitis: 40 mg/kg/8 h.For infection by CR GNB (meropenem MIC ≤ 8 mg/L):40 mg/kg/8 h infusion over 3 hours. | Decreases plasma concentration of valproic acidb |
| Imipenem (imipenem-cilastatin) (IV) | ≥3 mo: 15-25 mg/kg/6 h.<3 mo and ≥1.5 kg of weight, age:1-3 mo: 25 mg/kg/6 h<1 mo: dose based on weight, GA and PNA. Max: 4 g/day. | Use the highest dose in severe infection and/or infection by P. aeruginosa. | Do not use in case of CNS infection. Decreases plasma concentration of valproic acidbOff-label in age <12 months. |
| Ertapenem (IV) | >12 y: 1 g/24 h.3 m-12 y: 15 mg/kg/12 h (max: 500 mg/dose). | Consider 2 g/day and administration q12 h in children aged >12 y for combined therapy against CR GNB. | Decreases the plasma concentration of valproic acidbNot active against P. aeruginosa.Use in combination with meropenem for treatment of CR GNB infections only if pandrug-resistant isolate (scarce data). |
| Gentamycin (IV) | ≥1 mo: 5-7.5 mg/kg/24 h. < 1 mo: dose based on weight, GA and PNA. | Consider 7.5-10 mg/kg/24 h in patients with shock, pulmonary infection or cystic fibrosis. | Target plasma concentration: peak 6-12 μg/mL, trough <2 μg/mL. |
| Amikacin (IV) | ≥1 mo: 15-22 mg/kg/24 h. < 1 mo: dose based on weight, GA and PNA. | Consider 22–30 mg/kg/day in patients with shock, pulmonary infection or cystic fibrosis. | Target plasma concentration: peak 20-35 μg/mL, trough <5 μg/mL. |
| Colistin (colistimethate sodium)c (IV) | 75 000-150 000 IU/kg/day c/8-12 h (max: 10 800 000 IU/day). | Consider 150 000-250 000 IU/kg/day in patients with shock, pulmonary infection, severe UTI or cystic fibrosis. | We recommend a loading dose of 75 000-150 000 IU/kg in critically ill patients. |
| Tigecycline (IV) | ≥12 y: 50 mg/12 h.8-11 y: 1.2 mg/kg/12 h (max: 50 mg/12 h). | Consider higher loading and maintenance dose in patients with pulmonary infection, severe UTI, bloodstream infections or shock: ≥12 y: 100-200 mg loading dose followed by 100 mg/12 h.8-11 y: 2-3 mg/kg loading dose (max: 200 mg) followed by 2 mg/kg/12 h (max: 100 mg/12 h). | Restricted for children aged ≥8 y with infection by multidrug-resistant bacteria in the absence of other treatment options.Do not use in case of bloodstream infections or pneumonia, except as a last resort. |
| Fosfomycin (IV) | >12 y (>40 kg): 12-24 g/day q6-8 h 1-12 y (10-40 kg): 200-400 mg/kg/day q6-8 h.1-12 mo (≤10 kg): 200-300 mg/kg/day q8 h.<1 mo: dose based on weight, GA and PNA.Max: 8 g/dose; 24 g/day. | Consider using max dose in severe infections, especially those caused by strains with moderate susceptibility. | Monitor serum sodium concentrations and fluid balance due to high sodium content (330 mg per gram of fosfomycin) |
| Aztreonam (IV) | ≥1 mo: 120-150 mg/kg/day q8 h (max: 8 g/day).<1 mo: dose based on weight, GA and PNA. | Do not use as monotherapy in infections by AmpC- or ESBL-producing strains. | |
| Piperacillin-tazobactam (IV) | >12 y and/or ≥ 40 kg: piperacillin 2-4 g/dose q6-8 h.1 mo-12 y and/or <40 kg: piperacillin 350-400 mg/kg/day q6-8 h.<1 mo: dose based on weight, GA and PNA.Max: 16 g/day piperacillin. | Consider use of max dose and administration q6 h in severe or high-inoculum infections, considering extended infusion over 4 hours. | In treatment of infection by ESBL-producing Enterobacterales with documented in vitro susceptibility, administer the max dose q6 h by extended infusion over 4 h. |
| Cefepime (IV) | ≥2 mo: 50 mg/kg/dose q8 h (max: 2000 mg/dose)<2 m (off-label): dose based on weight, GA and PNA. | Consider extended infusion over 4 hours in severe or high-inoculum infections. | Approved for use starting at age 2 months. |
| Ceftazidime-avibactam (IV) | IV Infusion over 2 hours:6 m-18 y: <40 kg: 50 mg/kg of ceftazidime q8 h; ≥40 kg: 2 g of ceftazidime q8 h.3-6 mo: 40 mg/kg of ceftazidime q8 h (max 2 g ceftazidime q8 h). | Consider infusion over 3 hours in severe infections. | Currently approved by FDA starting at >3 months. Not approved by EMA before age 18 years. |
| Ceftolozane-tazobactam (IV) | IV infusion over 1 hour:<18 y: 20 mg/kg of ceftolozane q8 h (max: 1 g ceftolozane q8 h). | Consider increasing the dose in severe lung infections: 40 mg/kg of ceftolozane q8 h (max 2 g ceftolozane q8 h). | Currently not approved for age <18 years. Dose based on data from phase I and II trials in children. |
CNS, central nervous system; CR, carbapenem-resistant; EMA, European Medicines Agency; ESBL, extended spectrum β-lactamase; FDA, Food and Drug Administration; GA, gestational age; GNB, Gram-negative bacilli; IU, international unit; IV, intravenous; MIC, minimum inhibitory concentration; PNA, postnatal age; UTI, urinary tract infection.
Severe UTI: manifesting with sepsis, septic shock or other complications (such as renal abscess).
In 2017, the WHO developed a priority list of bacteria to guide the research and development of new antibiotics.10 Carbapenem-resistant (CR) Pseudomonas aeruginosa and Enterobacterales resistant to carbapenem or third generation cephalosporins led the list in the critical level. Vancomycin-resistant Enterococcus faecium and methicillin-resistant Staphylococcus aureus (MRSA) were given high priority. This position statement focuses on the management of infections by these bacteria, whose treatment currently poses a challenge in paediatric clinical practice. In the case of Enterococcus spp. due to their particularities in terms of intrinsic and acquired resistances, we will address the most frequent phenotypes.
Infections by Gram-positive cocciMethicillin-resistant Staphylococcus aureusThe main mechanism of resistance is the acquisition of a new penicillin-binding protein, known as PBP2a, which is encoded by the mecA gene.11 In microbiology laboratories, these strains are detected by testing the susceptibility to cefoxitin, by molecular detection of the mecA gene or by detection of PBP2a by immunochromatographic assay. This phenotype entails resistance to all β-lactam agents with the exception of the cephalosporins ceftaroline and ceftobiprole. Historically, there have been differences between the clones involved in community versus health care-associated infections, which differed in virulence and antimicrobial susceptibility, with community clones more frequently exhibiting susceptibility to clindamycin and production of the Panton-Valentine leucocidin toxin. However, in recent years, the introduction of community clones in hospitals and the spread of hospital clones to the community have blurred these differences.11
EpidemiologyIn Spain, community-acquired MRSA infections in children were first reported in 2006. At present, these strains account for 5%–10% of all S. aureus infections in children, and for as many as 25% of cases of community-acquired pneumonia (CAP).12 Most of the reported cases correspond to skin and soft tissue infections (SSTIs), followed in frequency by pneumonia and bloodstream infections.
Although in the past few years its prevalence has gradually decreased in other European countries, it has remained stable in Spain, with a prevalence of approximately 12% in blood culture isolates in children based on data from the European Centre for Disease Prevention and Control (ECDC).
The COSACO study has found a prevalence of colonization by MRSA in children in Spain of 1.4%, with 4.4% S. aureus isolates exhibiting methicillin resistance.13 It is worth noting that 17% of S. aureus isolates were resistant to clindamycin, a proportion that increased to 26% in the case of MRSA.
TreatmentThe first step should be adequate source control of the infection (Table 3). In case of persistent bacteraemia despite correct treatment, an active search of metastatic foci should be performed.
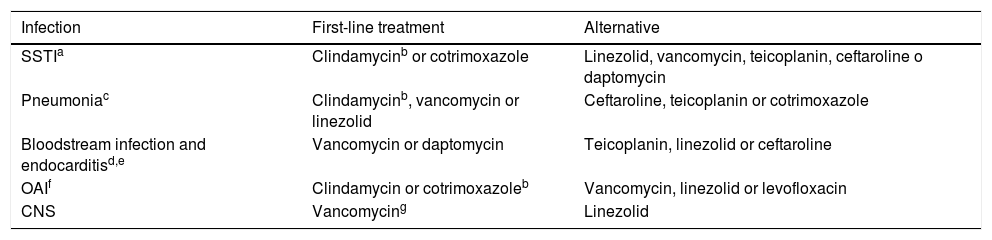
Treatment of infections caused by methicillin-resistant S. aureus.
| Infection | First-line treatment | Alternative |
|---|---|---|
| SSTIa | Clindamycinb or cotrimoxazole | Linezolid, vancomycin, teicoplanin, ceftaroline o daptomycin |
| Pneumoniac | Clindamycinb, vancomycin or linezolid | Ceftaroline, teicoplanin or cotrimoxazole |
| Bloodstream infection and endocarditisd,e | Vancomycin or daptomycin | Teicoplanin, linezolid or ceftaroline |
| OAIf | Clindamycin or cotrimoxazoleb | Vancomycin, linezolid or levofloxacin |
| CNS | Vancomycing | Linezolid |
CNS, central nervous system ; OAI, osteoarticular infection; SSTI, skin and soft tissue infection. Consider the order of appearance in the table as the order of preference for selection.
If oral treatment is indicated, since there is no commercially available oral solution of clindamycin in Spain, cotrimoxazole is a better alternative.
In case of persistent bacteraemia: if previous treatment with vancomycin, switch to daptomycin. If previous treatment with daptomycin, add ceftaroline or fosfomycin. If the patient has prosthetic devices, consider adding rifampicin. Persistent bacteraemia is defined as the persistence of positive results of blood culture 72 h or longer after initiation of adequate treatment (including central line removal) or the development of septic thromboembolic events or distant metastases of infection after 72 h of antibiotic therapy.
In case of endocarditis over a prosthetic valve, it is recommended: (vancomycin or daptomycin) + rifampicin + gentamycin.
In case of infection of an osteoarticular prosthesis with maintenance of the osteosynthesis material, add rifampicin. Some authors recommend adding it in a second phase targeted to treat the biofilm, after 7 days of initial treatment.15
Glycopeptide antibiotics (vancomycin or teicoplanin) are currently the first-line treatment for MRSA infections. However, their high binding to plasma proteins hinders their diffusion into the tissues. For this reason, it is essential to monitor their plasma concentrations to guarantee values that are adequate for treatment of severe infections. Unfortunately, in recent years there has been evidence of an increase in the minimum inhibitory concentration (MIC) of these antibiotics required for treatment of S. aureus isolates. In addition, there are concerns regarding the increased risk of treatment failure in strains with a vancomycin MIC of 1.5 mg/L or greater,14 so we recommend not using vancomycin if the MIC is 2 mg/L or greater, and in case of a MIC of 1.5 mg/L or greater (determined by Etest), we recommend considering alternatives after consulting with an expert.
Among the available options, clindamycin and linezolid inhibit toxin synthesis, so their use as monotherapy or in combination with other antimicrobials may be useful in case of infection by toxin-producing strains. Linezolid diffuses readily into tissue and allows sequential intravenous-to-oral therapy, although it can cause haematotoxicity and neurotoxicity with prolonged treatment.
Rifampicin exhibits excellent activity against biofilm, and its inclusion in the first-line antibiotherapy strategy is recommended in case of S. aureus infections associated with joint prosthesis.15 Nowadays, cotrimoxazole maintains activity against most MRSA isolates. Furthermore, it is distributed in Spain in the form of oral solution, which is a good option in cases of mild or moderate infections.
In cases of bloodstream infections or endocarditis due to MRSA with a vancomycin MIC of 1.5 mg/L or greater, daptomycin is a suitable alternative. This antimicrobial has bactericidal activity, is approved for use in children aged more than 1 year and is administered as a single dose per day. One of its disadvantages is that it is inhibited by pulmonary surfactant.
Ceftaroline is a fifth-generation cephalosporin approved for use from birth for treatment of SSTIs and CAP, with an excellent safety profile and strong activity against MRSA,16 although the data on its use in children are still scarce.
Enterococcus spp.Enterococcus genus is characterised by an intrinsic resistance to multiple antimicrobials, including nearly all cephalosporins, which makes treatment challenging. Furthermore, they can acquire additional resistance (for instance, to ampicillin or vancomycin),17 further reducing the treatment options.
E. faecalis is the most frequent bactera involved in these infections and it is nearly uniformly susceptible to aminopenicillins, being ampicillin the treatment of choice. E. faecium is second in frequency and is usually resistant to ampicillin and susceptible to vancomycin. E. gallinarum and E. casseliflavus, much less frequent, are intrinsically resistant to vancomycin (although not to teicoplanin), but usually susceptible to aminopenicillins.
Another relevant aspect of Enterococcus spp. is their intrinsic low-level resistance to aminoglycosides due to a deficient transport to the cytoplasm of the bacteria. However, when combined with another antimicrobial that acts on the cell wall (e.g. a β-lactam or glycopeptide), there is an increase in the penetration of the aminoglycoside, with a synergistic bactericidal effect, that is necessary for the treatment of severe infections (such as endocarditis, meningitis and bloodstream infections). Enterococcus spp. can acquire high-level resistance to aminoglycosides, in which case the synergistic effect disappears. The addition of ampicillin and ceftriaxone can also have a synergistic bactericidal effect, although only against E. faecalis.
Resistance to linezolid and daptomycin is currently very rare in Enterococcus spp. and mainly related to outbreaks in health care facilities or previous exposure to them.18
EpidemiologyThe prevalence of vancomycin resistance in E. faecium isolates from blood cultures in patients aged less than 18 years in the 2011–2012 period in 12 European countries was of 8.3%.19 However, based on data reported by the ECDC, in recent years the prevalence of resistance to vancomycin in Spain in invasive isolates from all age groups has ranged from 0.1% to 0.3% for E. faecalis and from 1.8% to 2.5% for E. faecium, remaining stable.
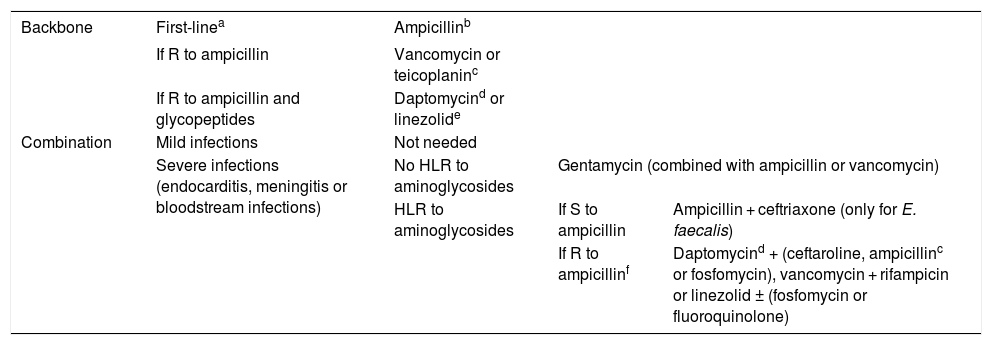
TreatmentWhenever the isolate is susceptible to ampicillin, this should be the first-line treatment (Table 4).4 The alternative would be a glycopeptide (vancomycin or teicoplanin). In case of severe infections, such as endocarditis, meningitis or bloodstream infections, we recommend the combination of 2 antibiotics to seek bactericidal synergy.20 The most frequently used combinations are ampicillin with gentamycin or ceftriaxone for infections by E. faecalis and vancomycin with gentamycin for infections by E. faecium.
Antimicrobial treatment of infections by Enterococcus spp.
| Backbone | First-linea | Ampicillinb | ||
|---|---|---|---|---|
| If R to ampicillin | Vancomycin or teicoplaninc | |||
| If R to ampicillin and glycopeptides | Daptomycind or linezolide | |||
| Combination | Mild infections | Not needed | ||
| Severe infections (endocarditis, meningitis or bloodstream infections) | No HLR to aminoglycosides | Gentamycin (combined with ampicillin or vancomycin) | ||
| HLR to aminoglycosides | If S to ampicillin | Ampicillin + ceftriaxone (only for E. faecalis) | ||
| If R to ampicillinf | Daptomycind + (ceftaroline, ampicillinc or fosfomycin), vancomycin + rifampicin or linezolid ± (fosfomycin or fluoroquinolone) | |||
HLR, high-level resistance (gentamycin MIC ≥ 500 mg/L); R, resistant; S, susceptible.
In case of urinary tract infection: may be treated with amoxicillin, fosfomycin, nitrofurantoin or fluoroquinolones.
E. faecium is usually resistant to ampicillin, so we do not recommend empirical treatment with this drug against this species.
In case of ampicillin MIC ≤ 64 mg/L, consider treatment with a high dose of ampicillin. Consider combined therapy in this case.
Daptomycin is not recommended for treatment of lung infections due to its inactivation by pulmonary surfactant. Furthermore, its penetration is poor in the central nervous system. Use higher doses (see Table 1) in case of severe infection.
If the selected combination includes aminoglycosides, in vitro testing must be performed before starting treatment to assess for a potential high-level resistance by determining the MIC. In case of high-level resistance (gentamycin MIC ≥ 500 mg/L or streptomycin MIC ≥ 2000 mg/L), alternative agents must be selected, such as ampicillin + ceftriaxone in case of E. faecalis. If the strain is resistant to ampicillin and glycopeptides, possible alternatives include daptomycin or linezolid.
E. faecalis and E. faecium can develop resistance to vancomycin and teicoplanin mediated by the gene vanA, or to vancomycin not to teicoplanin mediated by the gene vanB. However, there have been reports of resistance to teicoplanin emerging during treatment with this antimicrobial, and therefore its use is not recommended for treatment of these cases. The resistance to vancomycin of E. gallinarum, E. casseliflavus and E. flavescens is intrinsic and is mediated by the gene vanC, which only confers resistance to vancomycin but not to teicoplanin, so the latter could be used for treatment against these species.17
Infections by Gram-negative bacilliExtended-spectrum β-lactamase-producing EnterobacteralesExtended spectrum β-lactamases (ESBLs) are enzymes that can hydrolyse and thus cause resistance or reduced susceptibility to penicillins, oximino- β-lactams (cefotaxime, ceftriaxone, ceftazidime and cefepime) and monobactams (aztreonam), but not to cephamycins (cefoxitin) or carbapenems. They are generally inhibited in vitro by different β-lactamase inhibitors (such as clavulanic acid, tazobactam or avibactam), which helps differentiate them phenotypically from AmpC β-lactamases, which are only inhibited by avibactam.
Currently, the most common ESBLs are CTX-M type. Extended spectrum β-lactamase-producing strains usually contain other genes that confer resistance to aminoglycosides (mainly to gentamycin, and less frequently to amikacin), cotrimoxazole or fluoroquinolones, which further restricts therapeutic options.
EpidemiologyIn recent years, there has been evidence of a substantial increase in the prevalence of ESBL-producing Enterobacteralesin both hospital and community settings. A recent systematic review on ESBL-producing Enterobacterales isolates from paediatric blood cultures worldwide found an increase in the prevalence from 3.5% to 8% between 1996 and 2013.21
The prevalence in the paediatric age group in Spain exhibits a similar trend to the one observed globally. A study that analysed the epidemiology of community-acquired urinary tract infections (UTIs) in children aged less than 14 years in Spain in 2016 found a prevalence of ESBL-producing Enterobacterales of 3.2%.22 There are few data on infections outside the urinary tract in the paediatric population of Spain.
The most important factors associated to infection by ESBL-producing bacteria are previous hospitalization, recent surgery or recent antibiotic therapy, especially with third-generation cephalosporins, fluoroquinolones or carbapenems, and the presence of chronic disease.23 Furthermore, colonization by these strains could increase the risk of infection by them.24 A relevant phenomenon that is becoming increasingly known is the vertical transmission in mothers colonised by ESBL-producing Enterobacterales.25
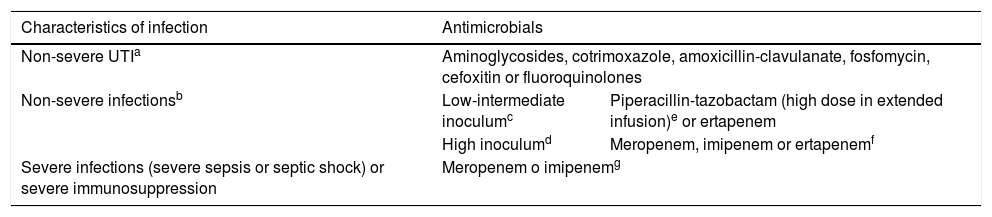
TreatmentThe selection of the antibiotic agent is based on the source and severity of the infection and the immune status of the patient (Table 5).8 Traditionally, carbapenems have been the antibiotics of choice for infections with this resistance profile. However, the increase in the prevalence of CR bacteria has underscored the need to identify carbapenem-sparing strategies to ensure a similar effectiveness without promoting the selection of resistant strains.
Treatment of infections by extended-spectrum β-lactamase-producing Enterobacterales. Adapted with consent from Gutierrez-Gutiérrez B.8
| Characteristics of infection | Antimicrobials | |
|---|---|---|
| Non-severe UTIa | Aminoglycosides, cotrimoxazole, amoxicillin-clavulanate, fosfomycin, cefoxitin or fluoroquinolones | |
| Non-severe infectionsb | Low-intermediate inoculumc | Piperacillin-tazobactam (high dose in extended infusion)e or ertapenem |
| High inoculumd | Meropenem, imipenem or ertapenemf | |
| Severe infections (severe sepsis or septic shock) or severe immunosuppression | Meropenem o imipenemg | |
UTI, urinary tract infection.
Consider sequential intravenous-to-oral therapy with any of the options included for non-severe UTI, according to the infection source, after improvement.
Low-intermediate-inoculum infections: central line-associated bloodstream infecion with removal of the central line, skin infections (drained in case of abscess), deep infections (e.g. intraabdominal) drained correctly or UTI.
High-inoculum infections: pneumonia, endocarditis, central nervous system infections and deep infections with inadequate drainage.
Although ESBL-producing bacteria often exhibit in vitro susceptibility to piperacillin-tazobactam, there have been reports of treatment failure, especially in cases of high-inoculum infections (pneumonia, undrained abscesses, etc). Data from observational studies have demonstrated the effectiveness of piperacillin-tazobactam, mainly in low-inoculum and non-severe infections and using an extended-infusion dosing strategy.8
The MERINO trial, which assessed mortality at 30 days from initiation of treatment for bloodstream infecions caused by Enterobacterales resistant to third generation cephalosporins (Escherichia coli and Klebsiella pneumoniae) did not demonstrate the non-inferiority of piperacillin-tazobactam compared to meropenem.26 However, several limitations of the study (such as deaths being mainly due to non-infectious causes, administration of piperacillin-tazobactam with a standard infusion protocol, etc) limit the generalizability of the results. A subsequent subanalysis found a similar mortality in case of bloodstream infections from a urinary source and non-severe infections, which was consistent with the findings of previous studies.8
Other antimicrobials (e.g. aminoglycosides, cotrimoxazole, amoxicillin-clavulanate, fosfomycin, cefoxitin or fluoroquinolones) may be an option in case of non-severe UTI and as sequential intravenous-to-oral therapy with a switch to the oral route after achieving control of the source of infection. Ertapenem is also a good alternative to meropenem in some cases of infection by ESBL-producing Enterobacterales, as it decreases antibiotic pressure on P. aeruginosa in addition to the advantages involved in the longer intervals between doses.
AmpC-producing EnterobacteralesAmpC β-lactamases can be chromosomal (intrinsic resistance) or encoded in plasmids (acquired resistance). Enterobacter spp. Citrobacter freundii complex, Serratia spp. Providencia spp, Morganella morganii and the species recently named Klebsiella aerogenes (previously known as Enterobacter aerogenes) all contain the gene for production of AmpC in their chromosomes and have intrinsic resistance to ampicillin, amoxicillin-clavulanate and first and second generation cephalosporins, but remain susceptible in vitro to third and fourth generation cephalosporins and piperacillin. The risk of induction of AmpC β-lactamase production is highest in Enterobacter species.27
In case of exposure to β-lactams, mainly cephalosporins and penicillins, the gene may be derepressed, leading to overexpression of the enzyme and producing the characteristic AmpC phenotype, which confers resistance to all β-lactams (except for cefepime, carbapenems and combinations with novel β-lactamase inhibitors, such as ceftazidime-avibactam). Plasmid AmpC β-lactamases can also be transferred between different Enterobacterales species, including those that do not have the AmpC gene in their chromosomes.
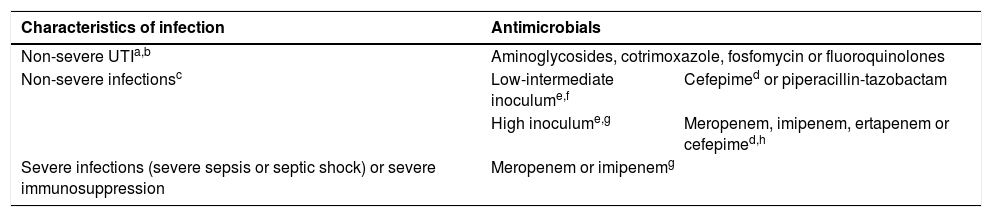
TreatmentIn case of severe infection by AmpC-producing bacteria, due to insufficient evidence, carbapenems are currently considered the most suitable option (Table 6).27 When feasible, targeted treatment with ertapenem could reduce antibiotic pressure on P. aeruginosa. Some non-carbapenem β-lactams, such as cefepime or piperacillin-tazobactam, are considered weak inducers, so they may be an alternative in certain circumstances with the use of high doses delivered through extended perfusion.28 We recommend against the use of piperacillin-tazobactam in high-inoculum infections or if the piperacillin-tazobactam MIC is greater than 8 mg/L, and against the use of cefepime in isolates for which the cefepime MIC is greater than 1 mg/L.29 Cefepime is usually not hydrolysed by AmpC β-lactamases, making it an excellent option. Several alternatives could be considered for treatment of non-severe UTI depending on the results of antimicrobial susceptibility testing (such as aminoglycosides, cotrimoxazole, fluoroquinolones, etc).
Treatment of infections by microorganisms with chromosomal or acquired AmpC.
| Characteristics of infection | Antimicrobials | |
|---|---|---|
| Non-severe UTIa,b | Aminoglycosides, cotrimoxazole, fosfomycin or fluoroquinolones | |
| Non-severe infectionsc | Low-intermediate inoculume,f | Cefepimed or piperacillin-tazobactam |
| High inoculume,g | Meropenem, imipenem, ertapenem or cefepimed,h | |
| Severe infections (severe sepsis or septic shock) or severe immunosuppression | Meropenem or imipenemg | |
UTI, urinary tract infection.
In case of non-severe UTI caused by Serratia spp, Providencia spp. or Morganella morganii, it is possible to treat with cefotaxime or ceftriaxone after confirming susceptibility to these agents with a close follow-up.
Use only in isolates with cefepime MIC ≤ 1 mg/L. In infections with intermediate or high inoculum administer every 8 h as extended infusion (over 4 h).
Consider sequential intravenous-to-oral therapy with any of the options included for non-severe UTI, according to the infection source, after improvement.
The development of carbapenem resistance is one of the most alarming phenomena within antimicrobial resistance, as it involves the loss of activity of a group of drugs with one of the broadest spectrums available. Furthermore, CR is frequently associated with resistance to other groups of antimicrobials (such as aminoglycosides or fluoroquinolones). In case of Enterobacterales, this resistance is usually due to production of carbapenemases, while the resistance of P. aeruginosa in Spain is usually due to non-enzymatic mechanisms (e.g. inactivation of porins and overexpression of efflux pumps).
Recent studies conducted in the United States have found an increase in the prevalence of infections by CR Gram-negative bacilli (GNB) in children, from 0% in 1999–2000 to 0.47% in 2010–2011 in the case of Enterobacterales,30 and from 9.4% in 1999 to 20% in 2012 in the case of P. aeruginosa.31
Currently, the prevalence of CR Enterobacterales in Spain is low, amounting to 4% of all invasive isolates of K. pneumoniae in 2017 (ECDC). Most of these cases occur in adults, and in children they mainly occur in the context of hospital outbreaks. The most prevalent carbapenemase in Spain is currently OXA-48-type, followed by metallo-β-lactamases (MBLs) and KPC β-lactamases.32 Due to the low circulation of these strains in children, their epidemiology has not been accurately established, although there is evidence of a predominance of MBLs of VIM class.33,34
Infections by these microorganisms have a higher mortality,3 and the risk factors for contracting them are similar to those observed in case of ESBL-producing GNB.35
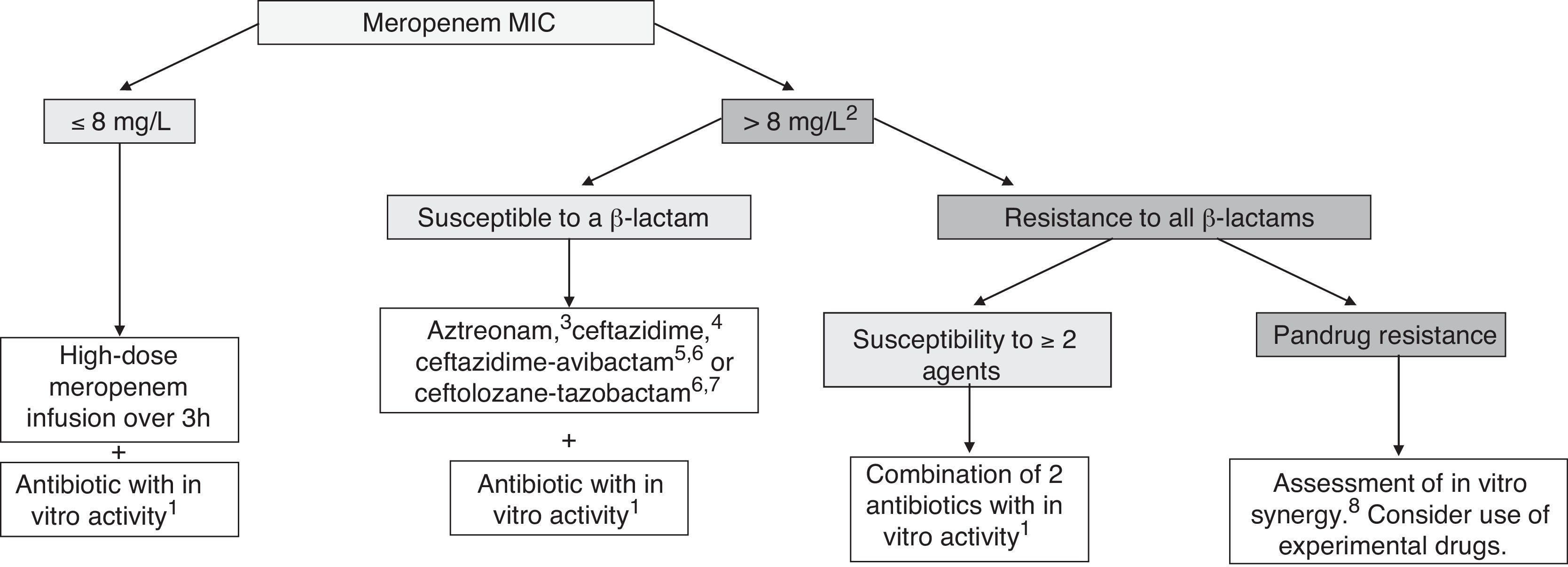
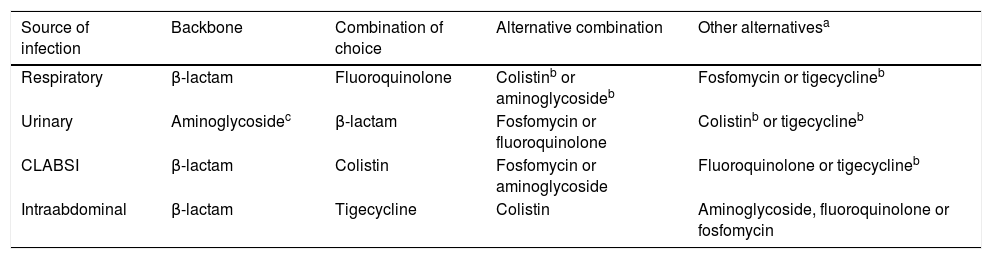
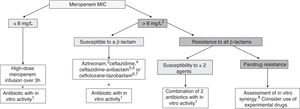
TreatmentCurrently, it is recommended combination therapy with 2 or more antibiotics with demonstrated activity against the isolated microorganism in a majority of cases (Fig. 1). The INCREMENT study, conducted in adults, propose the use of monotherapy in infections with a lower risk of severe disease.36 However, due to several factors, including the scales used or the lesser knowledge of the pharmacokinetics and pharmacodynamics of many antibiotics in children, the extrapolation of these findings to the paediatric population poses a challenge. Table 7 presents the combinations that may be used depending on the source of infection. For non-severe infections monotherapy could be individually considered.
Treatment of infections by carbapenem-resistant Gram-negative bacilli in children. Adapted from Hsu AJ.40
aPossible antibiotics: aminoglycoside, colistin, fosfomycin, fluoroquinolone or tigecycline. Choose the antimicrobial considering the source of infection and the susceptibility of the isolate (Table 7).
bConsider in case of a meropenem MIC > 4 mg/L, especially in severe infections.
cAztreonam may be used against GNB that produce MBLs (VIM, NDM or IMP) or OXA-48-type carbapenemases, if coproduction of ESBL or AmpC is not detected; if either of the latter are detected, consider use of aztreonam in combination with ceftazidime-avibactam if there is evidence of a synergistic in vitro effect.
dCeftazidime may be used in infection by OXA-48-producing GNB if coproduction of ESBL or AmpC is not detected.
eCeftazidime-avibactam may be used in infections by GNB with carpapenem resistance that is not mediated by MBLs.
fCurrently, ceftazidime-avibactam or ceftolozane-tazobactam, whose use would be off-label in children, should be restricted to severe infections in which the isolate has not exhibited susceptibility to other β-lactams, or in case of infection by a pandrug-resistant microorganism. Data from adults suggest that combined therapy is not neccesary when using these new antibiotics.
gCeftolozane-tazobactam may be used in infections by P. aeruginosa with carbapenem resistance that is not mediated by carbapenemases.
hSome combinations with which there is evidence of in vitro synergistic effect, but little clinical experience: ceftazidime-avibactam + aztreonam, meropenem + ertapenem, meropenem + fosfomycin, ceftazidime-avibactam + fosfomycin.
Treatment of infections by carbapenem-resistant Gram-negative bacilli based on the source of infection.
| Source of infection | Backbone | Combination of choice | Alternative combination | Other alternativesa |
|---|---|---|---|---|
| Respiratory | β-lactam | Fluoroquinolone | Colistinb or aminoglycosideb | Fosfomycin or tigecyclineb |
| Urinary | Aminoglycosidec | β-lactam | Fosfomycin or fluoroquinolone | Colistinb or tigecyclineb |
| CLABSI | β-lactam | Colistin | Fosfomycin or aminoglycoside | Fluoroquinolone or tigecyclineb |
| Intraabdominal | β-lactam | Tigecycline | Colistin | Aminoglycoside, fluoroquinolone or fosfomycin |
CLABSI, central line-associated bloodstream infecion.
Antimicrobials in the table will be used following the algorithm presented in Fig. 1, taking into account in vitro susceptibility of the isolate. Consultation with experts is recommended in every case.
In the treatment of intraabdominal infections, all antimicrobials with the exception of tigecycline and meropenem must be combined with an antimicrobial with anaerobicidal activity (such as metronidazole).
The use of optimised dosage of meropenem (double dose with extended infusion) achieves the desired pharmacodynamic target in isolates with a meropenem MIC of up to 8 mg/L,37 and has exhibited adequate effectiveness in observational studies.38,39 Therefore, we consider it the current treatment of choice, to be given in combination with a second antibiotic with demonstrated in vitro activity.7,40 In the case of isolates with meropenem MIC greater than 8 mg/L, the alternative of choice would be a β-lactam with demonstrated activity in antimicrobial susceptibility testing. If none of them are active against the isolate, other possible options include colistin, aminoglycosides, fosfomycin, fluoroquinolones or tigecycline.
The new β-lactam-β-lactamase inhibitor combinations currently distributed in Spain (ceftazidime-avibactam and ceftolozane-tazobactam) have not been authorised for use in the paediatric population. However, phase II clinical trials have proven its safety and efficacy in children, and ceftazidime-avibactam has been recently approved by the Food and Drug Administration (FDA) for use in children aged more than 3 months. Consequently, its compassionate use could be considered in patients with isolates with meropenem MIC greater than 8 mg/L with demonstrated susceptibility to this antibiotic, especially in cases of severe infection. Ceftazidime-avibactam has demonstrated activity against OXA-48- and KPC-producing bacteria, but not against those that produce MBL carbapenemases, while ceftolozane-tazobactam is not active against any carbapenemase-producing bacteria, but is active against P. aeruginosa with carbapenem resistance that is not mediated by carbapenemases.
In case of extremely resistant isolates, with few treatment options, we recommend that the microbiology laboratory evaluate in vitro the synergistic activity of different combinations (e.g., aztreonam-avibactam, meropenem-ertapenem, meropenem-fosfomycin).7 The combination of aztreonam and avibactam (currently, of aztreonam with ceftazidime-avibactam, as the combination has yet to be marketed), has proven effective against some MBL-producing strains that are resistant to aztreonam. Thus, this could be an option for treatment against pathogens with this type of carbapenemases in the absence of other alternative treatments.7
Although the current evidence on the use of inhaled antibiotics (e.g. colistin or tobramycin) is limited, they could be considered as an adjuvant to systemic antibiotic therapy in lung infections caused by CR GNB.
ConclusionIn recent years, there has been a progressive increase in the incidence of infections by MDR bacteria, and their treatment poses a significant challenge. To address this problem, it is essential to institute antibiotic stewardship programmes, active epidemiologic surveillance and adequate infection control protocols. The scarcity of therapeutic options, which is even higher in paediatric patients due to the scarce of clinical trials, makes the selection of an effective treatment difficult. The development of new antimicrobials will improve the prognosis of these infections. However, the rational use of antimicrobials is essential in order to preserve their effectiveness.
FundingDAA is funded by the Spanish Ministry of Health - Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union (FEDER) [Contrato Río Hortega CM18/00100].
Conflict of interestNone.
We thank the following for revising the manuscript: Natalia Mendoza Palomar (Paediatric Infectious Disease and Immunodeficiency Unit, Hospital Universitari Vall d'Hebron), M. Nieves Larrosa Escartín (Department of Microbiology, Hospital Universitari Vall d'Hebron), Silvia Manrique Rodríguez (Pharmacy Department, Hospital General Universitario Gregorio Marañón), Cecilia M. Fernández-Llamazares (Pharmacy Department, Hospital General Universitario Gregorio Marañón), Montserrat Giménez Pérez (Department of Microbiology, Hospital Universitario Germans Trias i Pujol) and Emilio Cendejas (Department of Microbiology and Parasitology, Hospital Universitario La Paz).
Please cite this article as: Aguilera-Alonso D, et al. Documento de posicionamiento de la Asociación Española de Pediatría-Sociedad Española de Infectología Pediátrica (AEP-SEIP) sobre el tratamiento de las infecciones por bacterias multirresistentes. An Pediatr (Barc). 2019;91:351.