Dexmedetomidine is a pharmacological option for sedation in children. In this study, the efficacy of intranasal dexmedetomidine to reduce preoperative anxiety in paediatric patients is compared with that of oral midazolam.
Materials and methodsA prospective, randomized, double-blind, controlled trial was conducted on children 2–12 years of age, randomly assigned to one of the following two groups: group A received premedication with oral midazolam and intranasal placebo, group B received intranasal dexmedetomidine and oral placebo. Anxiety was assessed with the modified Yale scale, and a risk analysis and number needed to treat was performed.
ResultsA total of 108 patients were included, 52 (48.1%) treated with dexmedetomidine, and 56 (51.9%) with midazolam. Anxiety was less frequent in the dexmedetomidine group at 60min (P=.001), induction (P=.04), and recovery (P=.0001). Risk analysis showed that dexmedetomidine reduced the risk of anxiety by 28% (RAR=0.28, 95% CI: 0.12–0.43) and to prevent one case of anxiety, four patients need to be treated with intranasal dexmedetomidine (NNT=4, 95% CI: 3–9). Changes in heart rate, mean arterial pressure, and oxygen saturation were statistically significant in the dexmedetomidine group, with no clinical consequences. There were no cases of bradycardia, hypotension or oxygen desaturation.
ConclusionsIntranasal dexmedetomidine premedication is more effective than oral midazolam to reduce preoperative anxiety in paediatric patients.
La dexmedetomidina es una opción farmacológica en la sedación del paciente pediátrico. En este estudio, se compara la eficacia de la dexmedetomidina intranasal versus midazolam por vía oral para disminuir la ansiedad preoperatoria en pacientes pediátricos.
Material y métodosSe realizó un ensayo clínico, doble ciego, en niños de 2 a 12 años de edad, asignados aleatoriamente a uno de los siguientes dos grupos: a) recibió medicación preanestésica con midazolam por vía oral y placebo intranasal; b) recibió dexmedetomidina intranasal y placebo por vía oral. Se evaluó la ansiedad con la escala de Yale modificada y realizamos el análisis de reducción de riesgo y un número necesario a tratar.
ResultadosSe estudió a 108 pacientes, 52 (48,1%) tratados con dexmedetomidina y 56 (51,9%) con midazolam. La ansiedad fue menos frecuente en el grupo de dexmedetomidina a los 60min (p=0,001), en la inducción (p=0,04) y en la recuperación (p=0,0001). El análisis de riesgo mostró que la dexmedetomidina redujo el riesgo de ansiedad en un 28% (RAR=0,28, IC del 95%, 0,12 a 0,43) y que para prevenir un caso de ansiedad es necesario tratar con dexmedetomidina intranasal a 4 pacientes (NNT=4, IC del 95%, 3 a 9). En el grupo de dexmedetomidina se registraron cambios estadísticamente significativos en la frecuencia cardiaca, la presión arterial media y la saturación de oxígeno, sin repercusión clínica; no se registraron casos de bradicardia, hipotensión ni desaturación de oxígeno.
ConclusionesLa premedicación con dexmedetomidina intranasal es más eficaz que el midazolam por vía oral para disminuir la ansiedad preoperatoria en pacientes pediátricos.
In a surgical procedure, the high anxiety levels that children experience during the preoperative period may be associated to negative medical, psychological, and social consequences. To reduce the distress of children and to facilitate the induction of anaesthesia, the child is given a sedative before going into the operating room.
The main goals of premedication in children are to facilitate a smooth separation from the parents and ease the induction of anaesthesia. Other effects that can be achieved with the pharmacological preparation of the patient are: amnesia, anxiolysis, prevention of psychological stress, and a reduction in the total anaesthetic requirements. The groups of patients that benefit most from premedication are toddlers and preschoolers, in whom separation anxiety is paramount; and also adolescents, who are sensitive about their body image and loss of control, patients with previous unpleasant hospital experiences, and patients who are unable to communicate or cooperate.1
Midazolam is the premedication most frequently used in children.2 It has been demonstrated that midazolam administered orally at 0.5mg/kg is efficacious in reducing anxiety and improving the conditions for induction of anaesthesia, but it has been associated with adverse effects such as agitation, paradoxical reaction, and negative postoperative behaviour changes.3 For this reason, other drugs and delivery routes are being explored. One of those drugs is dexmedetomidine.
Dexmedetomidine is a highly specific α2-adrenergic receptor agonist with sedative, anxiolytic, and analgesic properties, and a considerable sympatholytic effect. Its bioavailability when administered via the buccal route is 81.8%.4 In 1999 dexmedetomidine was approved in the United States by the Food and Drug Administration to be used in human patients as a short-term (<24h) sedative and analgesic in intensive care units.5 In healthy volunteers, intravenous dexmedetomidine has an action onset of 15min; it can also have systemic effects when administered by the transdermal, buccal, or intramuscular routes, with the latter two routes having showed bioavailabilities of 82% and 104%, respectively.6
It has been shown that in adult patients, intranasal administration of 1–1.5μg/kg of dexmedetomidine produces sedation within 45–60min, and has its peak effect at 90–105min. This is accompanied by mild to moderate changes in heart rate and arterial blood pressure.7
Recent studies in paediatric patients have shown that intranasal dexmedetomidine produces more sedation than oral midazolam.8 It has been used successfully in diagnostic radiology procedures, such as magnetic resonance imaging and computed tomography scans, and in invasive procedures, such as placement of venous lines in infants, bronchoscopy, laryngoscopy, cardiac catheterisation, and others.9,10 It has been used alone or in combination with other drugs to sedate paediatric patients that underwent different kinds of surgery.11–13 These studies did not report the effect of dexmedetomidine on anxiety, so the purpose of our study was to compare the efficacy of intranasal dexmedetomidine to oral midazolam in reducing preoperative anxiety in paediatric patients.
Materials and methodsWe conducted a double-blind controlled clinical trial. We included children 2–12 years of age of both sexes. Using the Power and Sample Size software, we calculated that a sample of 75 patients per group (n=150 patients) was needed to detect a difference of at least 15% in the prevalence of anxiety among groups, with an alpha of 0.05, a beta of 0.20, and a power of 0.80.
Patients were included by consecutive sampling of cases scheduled for elective surgery, ASA grade I, at the Hospital Regional Salamanca de Petróleos Mexicanos in Salamanca, Guanajuato, Mexico, between March 2009 and December 2011. We excluded patients with a history of allergy or hypersensibility to midazolam or dexmedetomidine, congenital heart disease or heart arrhythmias, mental retardation, or currently being treated with psychoactive medications.
The parents or guardian of the minors were informed of the purpose of the study and its procedures, and were asked to sign a consent form for participation.
The patients for whom we obtained consent to participate were assigned to one of 2 groups by means of a random number table with a secret sequence. Patients in the control group (midazolam) received oral midazolam, 0.5mg/kg, as preanaesthetic medication along with an intranasal placebo (0.9N isotonic saline solution). The study group (dexmedetomidine) received intranasal dexmedetomidine, 1μg/kg, as preanaesthetic medication, and an oral placebo (5% glucose solution). The drugs were administered 60min prior to induction of anaesthesia. Intranasal dexmedetomidine was prepared from the parenteral preparation (100μg/mL) and administered undiluted. The preparation and administration of medications were performed by a resident doctor in anaesthesiology that did not participate in clinical assessment, and the participating anaesthesiologists were blinded to the treatment. During surgery, all participants were monitored with class II (noninvasive) devices with electrocardiography, pulse oxymetry for monitoring oxygen saturation, and monitoring of blood pressure until they were discharged from the postanaesthetic care unit.
We assessed anxiety using the modified Yale scale,14 which determines the anxiety of the child based on a total of 22 items divided into 5 categories, each of which can contribute up to 4 or 6 points to the final score: activity (4), vocalizations (6), emotional expressivity (4), state of arousal (4), and use of parent (4). The scale was applied at 4 times: a baseline assessment prior to medication; 60min before induction; at time of induction of anaesthesia, and during post-emergence recovery. Patients were considered to have no anxiety for scores of 23.4–30, and to have anxiety for scores greater than 30.
We compared the groups by means of the chi-squared or the Student's t-tests as appropriate for the type of variable. We compared the prevalence of anxiety between groups with the chi-squared test with Yates’ correction or Fisher's test depending on the distribution of the data. We performed risk-reduction and intention-to-treat analyses.
During the preanaesthetic interview a week before the surgery, the parents or guardian of the minor were informed in detail of the characteristics of the study, its risks (respiratory depression with midazolam and haemodynamic alterations with dexmedetomidine) and possible benefits, and were asked to sign the informed consent form. The study protocol was submitted for approval to the Local Research Committee of the Hospital Regional Salamanca de PEMEX, and to the Research Committee of the Department of Medicine and Nutrition, and was accepted under the Registry N. 362-10. The medications used in the project were supplied by the hospital's pharmacy.
ResultsWe studied 108 patients aged 2–12 years (4 years, 95% CI: 3–5); the dexmedetomidine group included 52 participants (48.1%) and the midazolam group 56 participants (51.9%). The study included all patients undergoing elective surgery at the Hospital Regional Salamanca de Petróleos Mexicanos during the established period. We did not observe significant differences in age, anthropometric variables, or the baseline haemodynamic variables (Table 1).
Baseline characteristics of the population under study.
| Variable | Dexmedetomidine, n=52 | Midazolam, n=56 | p |
| Age (years) | 4 (3–5) | 4 (3–5) | 0.61 |
| Sex (female/male) | 28/24 | 24/32 | 0.25 |
| Weight (kg) | 16.0±5.6 | 17.4±7.12 | 0.43 |
| Height (m) | 72.4±40.3 | 71.9±41.9 | 0.96 |
| HR (beats/min) | 104.8±11.7 | 102.2±11.7 | 0.41 |
| MAP (mm Hg) | 67.8±7.8 | 67.9±8.9 | 0.95 |
| SpO2 (%) | 98.9±0.7 | 98.6±0.8 | 0.25 |
Abbreviations: HR, heart rate; MAP, mean arterial pressure; SpO2, oxygen saturation.
The values are expressed as mean±SD.
The types of surgery performed are shown in Table 2. The most frequent procedure in the dexmedetomidine group was inguinal hernia repair (53.5%), followed by amygdalectomy (15.3%) and umbilical hernia repair (11.5%); while in the midazolam group the most frequent procedure was circumcision (28.5%), followed by inguinal hernia repair (25.0%), and umbilical hernia repair in 10.7% of patients (χ2=22.7, P=.006).
Types of surgery performed in the two groups of the study.
| Surgery | Group | Total | |
| Dexmedetomidine N (%) | Midazolam N (%) | N (%) | |
| Amygdalectomy | 8 (15.3) | 6 (10.7) | 14 (12.9) |
| Circumcision | 4 (7.6) | 16 (28.5) | 20 (18.5) |
| Hypospadias repair | 1 (1.9) | 1 (1.7) | 2 (1.8) |
| Endoscopy | 0 | 2 (3.4) | 2 (1.8) |
| Synovial cyst excision | 0 | 1 (1.7) | 1 (0.9) |
| Inguinal hernia | 28 (53.8) | 14 (25.0) | 42 (38.8) |
| Umbilical hernia | 6 (11.5) | 6 (10.7) | 12 (11.1) |
| Orchidopexy | 2 (3.8) | 1 (1.7) | 3 (2.7) |
| Polyp resection | 0 | 2 (3.4) | 2 (1.8) |
| Other | 3 (5.7) | 7 (12.5) | 10 (9.2) |
Previous to treatment administration, anxiety levels were similar in both groups (χ2=1.5, P=.26); however, as shown in Table 3, anxiety was lower in the dexmedetomidine group at 60min (P=.001), at induction (P=.04), and during recovery (P=.0001). Risk analysis showed that 60min after administration, dexmedetomidine had reduced the risk of anxiety by 28%, and 4 patients needed to be treated with intranasal dexmedetomidine to prevent 1 case of anxiety (number needed to treat=4; 95% CI: 3–9).
Changes in anxiety following treatment.
| Surgical time | DexmedetomidineWith/without anxiety | MidazolamWith/without anxiety | RR (95% CI) | RRR (95% CI) | RAR (95% CI) | NNT (95% CI) |
| Baseline | 2/50 | 2/54 | 1.08 (0.16–7.3) | – | – | – |
| 60min | 6/46 | 22/34 | 0.29 (0.13–0.67) | 0.71 (0.33–0.87) | 0.28 (0.12–0.43) | 4 (3–9) |
| Induction | 8/44 | 18/38 | 0.48 (0.23–1.01) | 0.52 (0.01–0.77) | 0.17 (0.01–0.32) | 6 (4–93) |
| Recovery | 2/50 | 30/26 | 0.07 (0.02–0.29) | 0.93 (0.71–0.98) | 0.50 (0.36–0.64) | 3 (2–3) |
Abbreviations: CI, confidence interval; NNT, number needed to treat; RR, risk reduction; RRR, relative risk reduction.
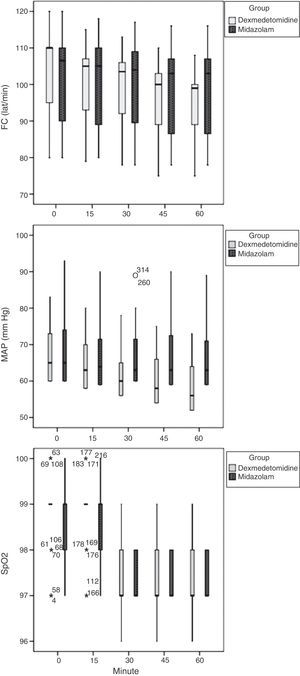
Data for the noninvasive monitoring carried out during surgical procedures are shown in Fig. 1. Children in the dexmedetomidine group showed a statistically significant reduction in heart rate (F=7.6, P=.001), mean arterial pressure (F=12.4, P=.001), and oxygen saturation (F=7.3, P=.001). Analysis of variance (ANOVA) with Tukey's post hoc test showed that on average heart rate had decreased by an average of 8 beats per minute (95% CI: 2–13) 45min after administration of the drug (P=.001), while arterial blood pressure had decreased by an average of 5mm Hg (95% CI: 1–8) by 30min post-treatment (P=.005), and oxygen saturation had decreased an average of 1.5% (95% CI: 1.1–1.9) at 30min post-treatment (P=.001). There were no cases of bradycardia, hypotension, or oxygen desaturation.
The midazolam group did not show significant changes in heart rate (P=.47) or mean arterial pressure (P=.78), while showing a significant change in oxygen saturation (P=.001) that had dropped by 1.2% on average (95% CI: 0.8–1.5) 15min after administration. There were no cases of bradycardia, hypotension, or oxygen desaturation in this group.
DiscussionVarious drugs have been used for premedication in paediatric patients to achieve hypnosis and anxiolysis, which are important factors in facilitating non-distressful separation from parents and a safe and non-traumatic induction of anaesthesia. Midazolam and clonidine have been used for oral delivery.15 Midazolam has been associated with adverse effects including agitation, paradoxical reaction, and negative postoperative behavioural changes, making it less safe for use in children, and clonidine is not available in Mexico. Respiratory depression can be a complication resulting from sedation or preanaesthetic medication with these drugs, and it has been observed in 5.5% of children.
Dexmedetomidine is an alpha-2-agonist drug that has been proven useful as an sedative, analgesic, anxiolytic, and anaesthetic coadjuvant both in adults and children.7 Recent studies have assessed the efficacy of intranasal dexmedetomidine as a sedative in children with promising results,8,12,13 although they have not assessed the anxiolytic effects of this drug.
An intranasal preparation of dexmedetomidine is not available yet. In this study, we used the parenteral preparation directly, without diluting it, which was done in other studies.8 These variations could lead to significant errors when comparing study results. By not diluting the drug, it was administered in small volumes that were better accepted by patients. We did not observe any adverse haemodynamic or ventilatory effects.
During the study design we calculated that we needed a sample size of 75 patients per group (n=150), and during the period of the study we only managed to enrol 108 participants. This does not mean that the favourable results should be disregarded, as the sample in the study achieved an alpha of 0.05, a beta of 0.20, and a power of 0.89.
In our series, intranasal administration of dexmedetomidine produced a statistically significant reduction in heart rate, mean arterial blood pressure, and oxygen saturation, effects that have been described for the pharmacokinetics of α-2-agonists5; but the average changes of these variables did not have relevant clinical consequences. This study shows that intranasal dexmedetomidine can be a useful pharmacological option for preanaesthetic medication in paediatric patients with no haemodynamic or ventilatory adverse effects.
ConclusionsIntranasal dexmedetomidine is a more efficacious premedication oral midazolam to reduce preoperative anxiety in paediatric patients.
RecommendationsUse of intranasal dexmedetomidine is recommended for preanesthetic medication in paediatric patients; the drug is easily administered, is a flavourless solution, and is given in small volumes.
Conflicts of interestThe medications used in the study were provided by the pharmacy of the Hospital Regional Salamanca de Petróleos Mexicanos.
The authors have no conflicts of interest to declare.
Please cite this article as: Linares Segovia B, García Cuevas MA, Ramírez Casillas IL, Guerrero Romero JF, Botello Buenrostro I, Monroy Torres R, et al. Medicación preanestésica con dexmedetomidina intranasal y midazolam oral como ansiolítico. Un ensayo clínico. Anales de Pediatría. 2014;81:226–231.