To describe preventive, diagnostic and therapeutic strategies regarding necrotising enterocolitis in Spain and to identify the strengths, areas of further improvement, and future research lines.
MethodsTwo questionnaires on the management of preterm infants less than 32 weeks, at risk of, or with diagnosed necrotising enterocolitis, were distributed among selected representatives of the surgeons and neonatologists of the Spanish Neonatal Network (SEN1500) participant hospitals with a Paediatric Surgery Department.
ResultsPercentage of response was 77.1% of contacted surgeons and 88.6% of neonatologists. There is a written protocol on the diagnosis and medical management of necrotising enterocolitis in 52% of the hospitals, and as regards surgical treatment in 33%. There is wide access to donor bank milk and to staff dedicated to breastfeeding promotion (87%). On the contrary, only 52% of the centres perform delayed cord clamping, and probiotics are used in just 23%. The use of abdominal ultrasound is increasing. There are no large differences as regards duration of antibiotic use and bowel rest, whereas there was as regards antibiotic selection, surgical indication, and type of intervention.
ConclusionsAs regards prevention, delayed cord clamping and extended access to donor milk are two possible aspects of further improvement. The observed discrepancies noted in diagnostic and therapeutic aspects are common in precisely the areas where evidence in the literature is weakest.
Describir la prevención, diagnóstico y tratamiento de la enterocolitis necrosante en hospitales españoles e identificar puntos fuertes, áreas de mejora y líneas de investigación pendientes.
MétodosSe realizaron 2 encuestas sobre manejo de pacientes en riesgo o diagnóstico de enterocolitis necrosante en recién nacidos pretérmino menores de 32 semanas, distribuidas entre representantes de los cirujanos pediátricos y neonatólogos de los centros participantes en la red española SEN 1500 con Servicio de Cirugía Pediátrica.
ResultadosEl porcentaje de respuestas fue del 77.1% y del 88.6% entre los cirujanos y neonatólogos contactados, respectivamente. El 52% de los hospitales dispone de un protocolo de diagnóstico y manejo médico de la enterocolitis y el 33% uno sobre tratamiento quirúrgico. El acceso a leche de banco y disponer de personal dedicado a la promoción de la lactancia materna es común (87%), por el contrario, la ligadura tardía de cordón solo se realiza en el 52% de los centros y en un 23% se administran probióticos. La ecografía abdominal está cada vez más extendida. No hay grandes diferencias en cuanto a la duración de los antibióticos y del reposo intestinal, pero si en cuanto a los antibióticos seleccionados, la indicación quirúrgica y el tipo de intervención.
ConclusionesLa implementación de la ligadura tardía de cordón y la extensión del acceso a leche de banco son áreas de mejora en el aspecto preventivo. En cuanto al diagnóstico y tratamiento existe una gran división que afecta precisamente a las áreas donde la evidencia en la literatura es menor.
Necrotising enterocolitis (NEC) is the most severe gastrointestinal complication in preterm (PT) infants. The mortality of enterocolitis is as high as 50% in some case series, and it may have a significant impact on the neurodevelopment of survivors.1,2 In the past 5 years, the incidence of NEC in Spain has remained stable according to the annual morbidity and mortality reports of the Very Low Birth Weight Infant Network (SEN1500, https://www.seneo.es/Comisiones-y-grupos-de-trabajo/Redes-neonatales/SEN1500). Between January 2013 and December 2017, 6.8% (range, 6%–7.3%) of newborns with birth weights of less than 1500 g received a diagnosis of enterocolitis each year.3
In recent decades there have been significant advances in our understanding of the pathogenesis, epidemiology, diagnosis and management of NEC. Notwithstanding, there are still controversial areas in the management of PT newborns at risk or with a diagnosis of NEC, as evinced by a review of various surveys at the international level.4–6
The objectives of our study were: (1) to describe the preventive measures and the strategies used for diagnosis and management in Spanish hospitals, (2) to identify strengths and areas for improvement, and (3) to identify potential areas for future clinical research.
Material and methodsWe conducted a cross-sectional study for which we developed 2 separate questionnaires on the measures used for prevention, diagnosis and management of NEC in PT infants (delivered before 32 weeks’ of gestational age) in Spain. We submitted the questionnaires to a representative of the Departments of Paediatric Surgery and Neonatology in each of the 35 hospitals members of the SEN 15007 that have both of these departments (Appendix A Supplemental material). Tables 1 and 2 detail the items that comprised the questionnaires designed for neonatologists (20 items on preventive, diagnosis and medical treatment measures) and paediatric surgeons (14 items on the indications for surgery, surgical treatment and postoperative care), respectively. We distributed the questionnaires electronically (https://www.google.com/intl/es/forms/about/) in May and June of 2019. We expressed the results as counts and percentages and represented them as pie charts.
Questionnaire distributed to the neonatologists of the selected hospitals.
| How many cases of necrotising enterocolitis (Bell stage II–III) in preterm newborns <32 weeks’ gestational age does your hospital manage each year? |
| Preventive measures |
| Is delayed cord clamping routinely performed at birth in stable preterm newborns <32 weeks in your hospital? |
| Is umbilical cord milking routinely performed at birth in stable preterm newborns <32 weeks in your hospital? |
| Does your hospital have a written protocol for enteral nutrition in preterm newborns (including timing of initiation and schedule of escalation)? |
| Does your hospital have accredited staff partially or exclusively dedicated to the promotion of breastfeeding? |
| Is donor human milk available in your hospital? |
| Do you routinely administer probiotics to preterm newborns in your hospital? |
| If you do, what probiotic is currently used in your hospital? |
| In case your hospital uses probiotics, what is the usual time of initiation? |
| • Within 48 h of birth, whether or not enteral feeding has been initiated |
| • As soon as possible but not until enteral feeding has started |
| Does your hospital have a written protocol to establish the indication of antacid drugs (ranitidine, omeprazole or similar)? |
| When it comes to the duration of empirical antibiotherapy initiated at birth in preterm infants, if the results of blood culture are negative: |
| • Antibiotherapy is discontinued at 48 h if the patient is stable and maintained if the patient is unstable (clinical suspicion of sepsis with negative culture) |
| • Antibiotherapy is maintained from 2–7 days if the patient is stable, regardless of the results of blood culture at 48 h |
| Diagnosis and medical treatment |
| Does your hospital have a written protocol for the diagnosis and management of necrotising enterocolitis? |
| What is the initial imaging test performed in your unit in case of suspected necrotising enterocolitis? |
| • Plain abdominal X-ray in every case, abdominal ultrasound never or seldom used for initial evaluation |
| • Abdominal ultrasound in every case, plain abdominal X-ray never or seldom done as initial imaging test |
| • Plain abdominal X-ray and abdominal ultrasound for initial evaluation in all or nearly all cases |
| If abdominal ultrasound is not used as an initial imaging test: In your hospital, in patients with necrotising enterocolitis, abdominal ultrasound: |
| • Is used as a secondary imaging test in the diagnosis or follow-up of patients in select cases |
| • Is never or seldom used in patients with necrotising enterocolitis |
| Once the diagnosis of enterocolitis (Bell II-III) is confirmed, radiological follow-up (plain abdominal radiograph and/or abdominal ultrasound) is performed: |
| • Regularly, independently of the clinical condition and course of disease in the patient |
| • Only in case of changes in the condition of the patient or the course of disease |
| In your hospital, regional abdominal oxygen saturation is monitored with NIRS in patients with suspected (stage i) or confirmed (stage ii-iii) necrotising enterocolitis: |
| • Never |
| • in very select cases |
| • Routinely |
| In case of confirmed necrotising enterocolitis (Bell stage II-III) how many antibiotics are prescribed to initiate therapy in your hospital? |
| • One, covering gram-negative, gram-positive and anaerobic bacteria (e.g. piperacillin-tazobactam) |
| • Two, covering gram-negative and gram-positive bacteria |
| • Three, covering gram-negative, gram-positive and anaerobic bacteria in every case, independently of Bell stage |
| • Three, covering gram-negative, gram-positive and anaerobic bacteria only in case of stage III or confirmed perforation |
| In patients with confirmed enterocolitis (Bell stage II-III) that do not require surgery, what is the usual duration of antibiotherapy in absence of complications? |
| • <7 days |
| • 7−10 days |
| • >10 days |
| For gastrointestinal decompression in patients with necrotising enterocolitis, do you routinely connect the naso/orogastric tube to a continuous or intermittent suction unit? |
| • Yes, it is part of the first-line treatment of patients with enterocolitis in our hospital |
| • Yes, but only in select cases, not routinely |
| • No, never or exceptionally, in these patients the tube is usually left open to allow gravity drainage |
| In patients with confirmed enterocolitis (Bell stage II-III) that do not undergo surgery, what is the usual duration of complete bowel rest in absence of complications? |
| • <7 days |
| • 7−10 days |
| • >11 days |
Questionnaire distributed to the paediatric surgeons of the selected hospitals.
| How many cases of necrotising enterocolitis in preterm newborns (gestational age <32 weeks) are managed surgically, including insertion of drains, in your hospital each year? |
| • 0–10 |
| • 11−20 |
| • >20 |
| Indications for surgery |
| Does your hospital have a written protocol for the surgical management of necrotising enterocolitis? |
| Do you think that indications for surgery in your hospital have changed in the past 10 years? |
| • No, surgery is performed in patients with perforation/pneumoperitoneum and the indication for lack of clinical improvement despite adequate medical treatment in absence of pneumoperitoneum continues to be secondary |
| • Yes, surgery is indicated at increasingly early stages, even in the absence of intestinal perforation/pneumoperitoneum if patients do not improve despite adequate medical care |
| • Yes, management is increasingly conservative in patients that do not improve despite adequate medical treatment and fewer patients undergo surgery, with surgery practically restricted to patients with confirmed perforation |
| Do you systematically use a pre-established score to assess the level of metabolic decompensation of the patient to determine whether surgery is indicated? |
| In your hospital, in patients with necrotising enterocolitis, the abdominal ultrasound scan : |
| • Is routinely used in the preoperative evaluation by most surgeons in your department |
| • Is not considered useful to determine the indication of surgery by most surgeons in your department |
| In your hospital, in the neonatal intensive care unit, laparoscopy is used in the evaluation of patients with necrotising enterocolitis that are candidates for surgical intervention: |
| • In select cases |
| • Never or exceptionally |
| Surgical management |
| In case laparotomy is performed, if focal disease is confirmed but intestinal resection is required, in a patient that weighs <1000 g: |
| • A diverting ostomy is the option preferred by most surgeons in your department |
| • Primary anastomosis is the option preferred by most surgeons in your department |
| In case laparotomy is performed, if focal disease is confirmed but intestinal resection is required, in a patient that weighs >1000 g: |
| • A diverting ostomy is the option preferred by most surgeons in your department |
| • Primary anastomosis is the option preferred by most surgeons in your department |
| In patients with necrotising enterocolitis and confirmed intestinal perforation (pneumoperitoneum) that are unstable: |
| • Primary peritoneal drainage with/without deferred laparotomy is the initial approach preferred by most surgeons in your hospital |
| • Laparotomy is the initial approach used by most surgeons in your hospital |
| In patients with necrotising enterocolitis without evidence of intestinal perforation (pneumoperitoneum) that do not improve despite adequate medical treatment and are unstable, if the decision is made to perform surgery: |
| • Peritoneal drainage with/without deferred laparotomy is the initial approach used by most surgeons in your hospital |
| • Laparotomy is the initial approach used by most surgeons in your hospital |
| If an enterostomy is performed, a contrast study is done to rule out stenosis before the stoma reversal procedure: |
| • Routinely |
| • Exceptionally or in select cases |
| How early after the initial surgery is enterostomy reversal considered in patients with a favourable response? |
| • Prior to discharge independently of age |
| • Before 6 months |
| • Before 1 year |
| • Before 2 years |
| In case of surgical management, what is the typical duration of antibiotherapy in a patient without postoperative complications? |
| • <7 days |
| • 7−10 days |
| • >1 day |
| In case of surgical management, how long is complete bowel rest maintained after surgery if there are no postoperative complications? |
| • <7 days |
| • 7−10 days |
| • >10 days |
We received responses from 27 (77%) of the paediatric surgeons and 31 (89%) of the neonatologists to whom we sent the questionnaires. Most of the hospitals selected for the survey manage fewer than 10 patients with enterocolitis each year (n = 30 hospitals, 97%). Only one hospital reported managing between 11 and 20 cases a year. The management of these patients is only partially standardised. Sixteen hospitals (52%) had a protocol for diagnosis and conservative treatment of NEC, and 9 (33%) had a protocol for surgical management of NEC.
Preventive measuresSixteen hospitals (52%) practiced delayed cord clamping in PT newborns, while only 3 (10%) practiced umbilical cord milking (2 hospitals in stable patients that did not need resuscitation, and 1 hospital also in unstable patients that require some form of resuscitation).
Twenty-five (81%) of surveyed hospitals reported having an enteral nutrition protocol for PT infants (which included the timing of initiation and the pace of escalation) and 3 (10%) a protocol on the use of antacid drugs. Twenty-seven hospitals (87%) had accredited lactation consultants on staff dedicated exclusively or part time to promotion of exclusive breastfeeding and had access to banked donor human milk.
Administration of probiotics was not a routine measure in 24 hospitals (77%). In fact, only 3 (10%) of surveyed hospitals administered probiotics routinely, and the remaining 4 hospitals (13%) only administered them within the framework of a clinical trial. Two of the hospitals that practiced routine administration of probiotics used a commercial combination of Bifidobacterium bifidum and Lactobacillus acidophilus, while the third one administered Lactobacillus reuteri. When it came to the hospitals participating in clinical trials, 3 used a combination of Bifidobacterium breve and Lactobacillus fermentum and the fourth one used a combination of Bifidobacterium longum and Lactobacillus salivarius. In every instance, administration of probiotics started as soon as possible after initiation of enteral feedings.
Most hospitals (n = 25; 81%) discontinued empirical antibiotherapy in patients at risk of early-onset neonatal sepsis at 48 h if the patient was stable, and continued treatment after 48 h if the patient was unstable (clinical suspicion of sepsis in patients with negative cultures). The other 6 hospitals (19%) maintained antibiotherapy for 2–7 days in stable patients regardless of the results of blood cultures at 48 h.
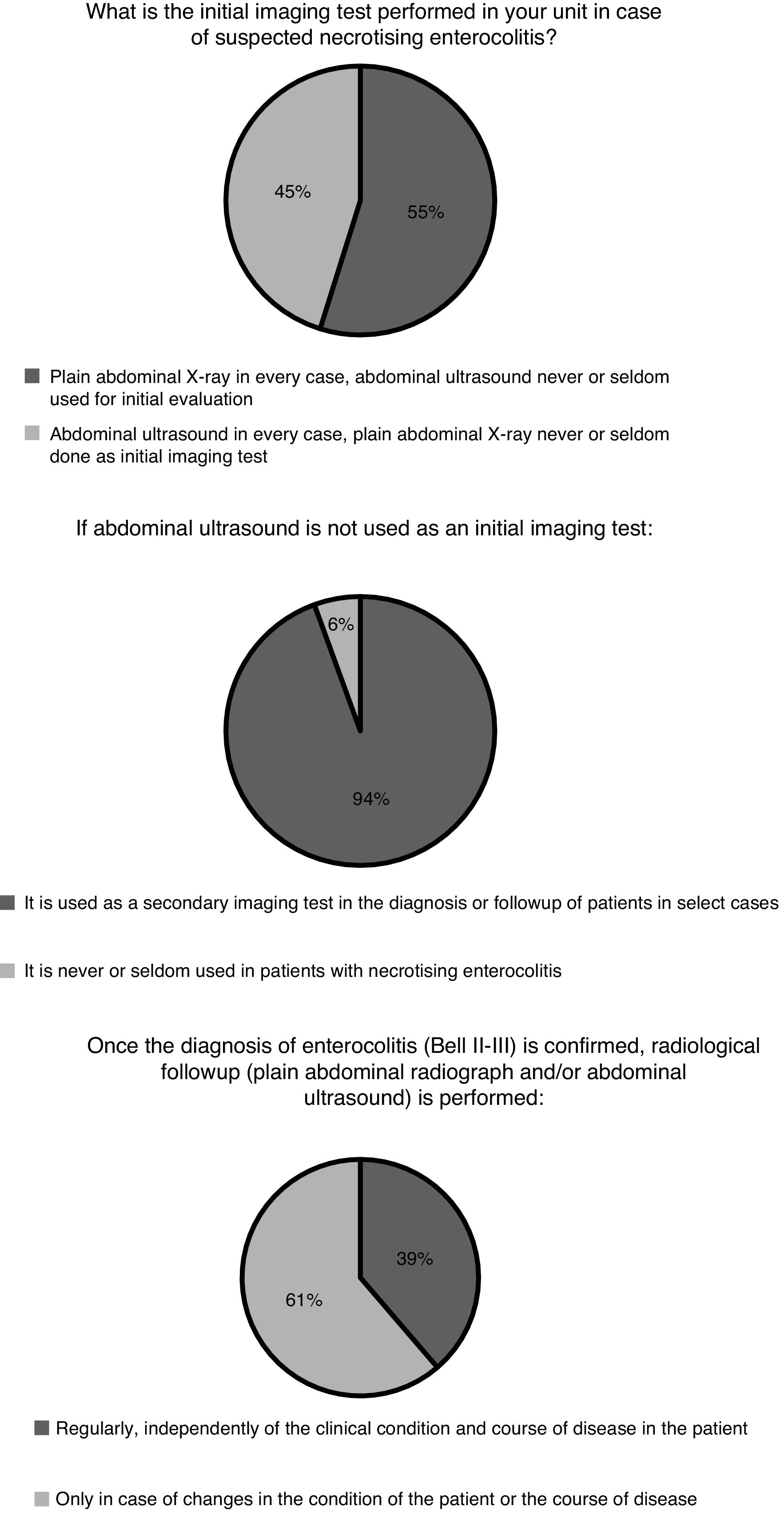
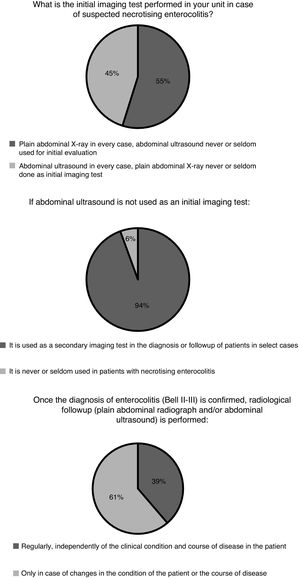
Diagnosis and medical treatmentThe indication for specific imaging tests for diagnosis and follow-up and the frequency of these tests varied between departments (Fig. 1). The most frequent test was the plain abdominal X-ray. The use of near-infrared spectroscopy (NIRS) monitoring in patients with suspected or diagnosed NEC was relatively widespread in Spanish neonatal units. Eight hospitals (26%) reported using it routinely and 10 (32%) occasionally in select cases. The remaining 13 (42%) did not use NIRS.
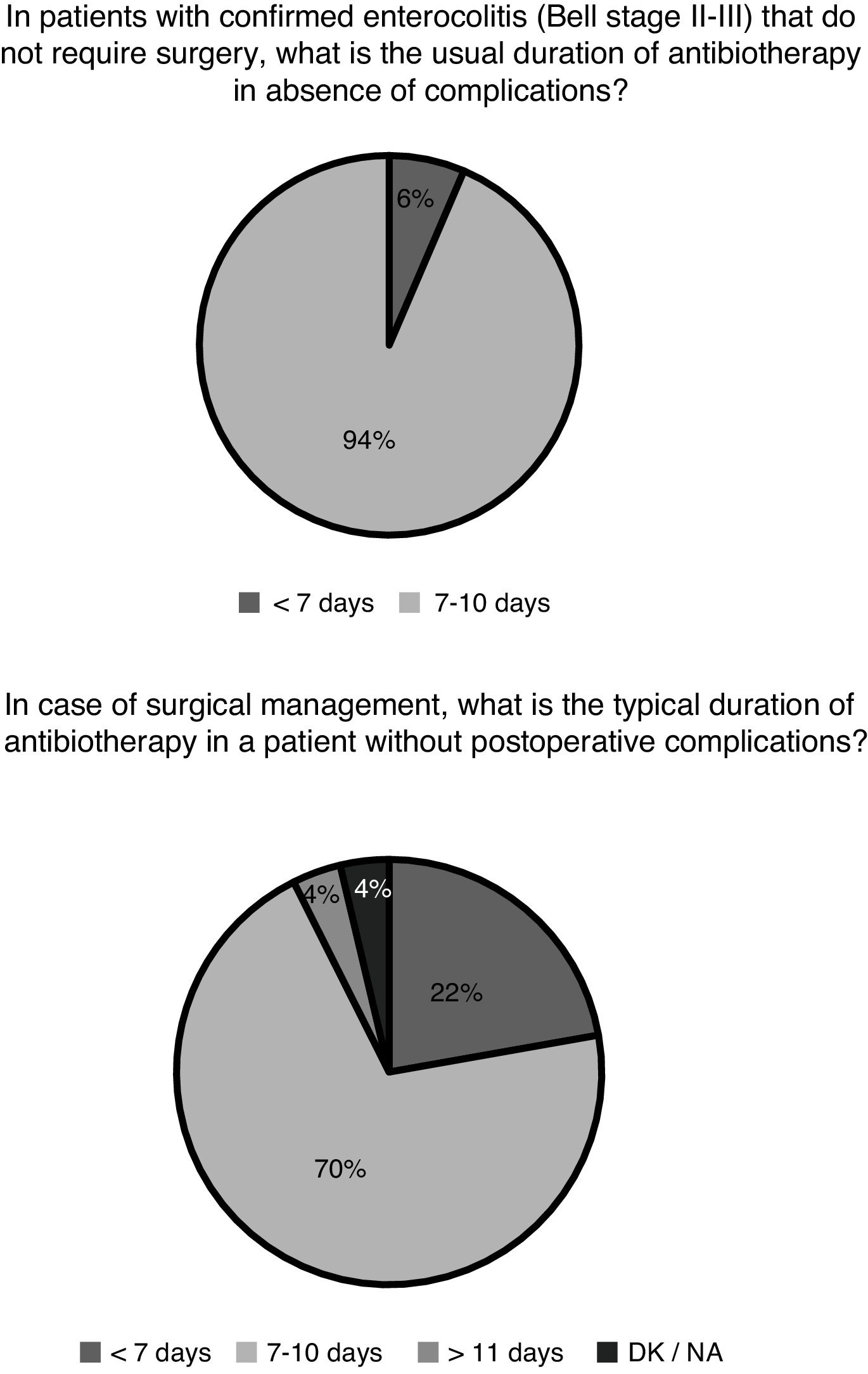
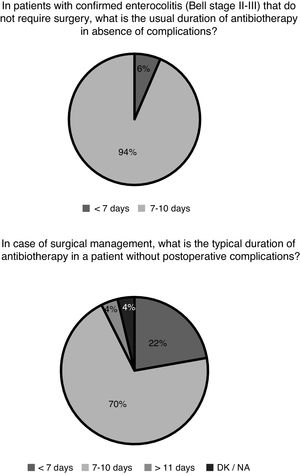
After confirmation of enterocolitis (Bell stage II or III), in patients that did not require surgical management, enteral nutrition was reintroduced after 7–10 days in 21 hospitals (70%) and in fewer than 7 days in 9 hospitals (30%); 1 hospital did not provide a response. Gastrointestinal decompression was also part of the standard care of patients with NEC. However, we found discrepancies when we asked whether connection to a suction unit was required for optimal decompression. Five of the hospitals (16%) routinely connected the naso/orogastric tube to a continuous or intermittent suction unit, and 3 (10%) did so in select cases. Lastly, we also found heterogeneity in the selection of antibiotic agents: 12 hospitals (39%) used 2 antibiotic agents to cover gram-negative and gram-positive bacteria, adding only a third to cover anaerobic bacteria in case of stage III NEC or confirmed perforation. Conversely, 8 hospitals (26%) always prescribed 3 antibiotic agents to cover gram-negative, gram-positive and anaerobic bacteria regardless of staging. Ten hospitals (32%) used only 2 antibiotic agents to cover gram-negative and gram-positive bacteria, and only 1 hospital (3%) reported using a broad-spectrum antibiotic covering gram-negative, gram-positive and anaerobic bacteria as monotherapy (e.g. piperacillin-tazobactam). Fig. 2 presents the durations of antibiotherapy in patients with medical and surgical enterocolitis.
Indications for surgeryIn 81% (n = 22) of surveyed hospitals, the indications for surgery had changed in the past 10 years. In 44% (n = 12) the changes involved increasing conservative treatment in patients that did not improve despite adequate medical care with a reduction in the proportion managed surgically, an approach that was reserved for patients with confirmed perforation; in 37% (n = 10) the changes went in the opposite direction, with surgical treatment indicated at earlier stages and even in the absence of intestinal perforation/pneumoperitoneum in patients that did not improve with adequate medical care. In the remaining 19% (n = 5), the indications had not changed (surgical management mainly indicated in patients with perforation/pneumoperitoneum, with a lack of clinical improvement despite adequate medical treatment in absence of pneumoperitoneum remaining a secondary indication). In 17 hospitals (63%), eligibility for surgery was determined considering the results ofan abdominal ultrasound as part the routine preoperative evaluation, while only 3 hospitals (11%) used a preestablished score to calculate the degree of metabolic decompensation to establish whether surgery was indicated. The use of laparoscopy to evaluate potential candidates for surgery was also exceptional. Twenty-two hospitals (81%) reported never using it or using it rarely, while 5 hospitals (19%) reported using it in select cases.
Surgical treatmentOnce eligibility for surgery has been established, the surgical strategy varies depending on the weight and clinical stability of the patient. In 63% of hospitals (n = 17), most surgeons decided on primary peritoneal drainage with or without deferred laparotomy for unstable patients with evidence of perforation, compared to 37% of hospitals (n = 10) where most would perform a primary laparotomy even in this subset of patients. In the case of unstable patients without pneumoperitoneum but that do not improve despite adequate medical care, 52% of hospitals (n = 14) would practice primary peritoneal drainage with or without deferred laparotomy, compared to 48% (n = 13) that would more frequently perform a primary laparotomy.
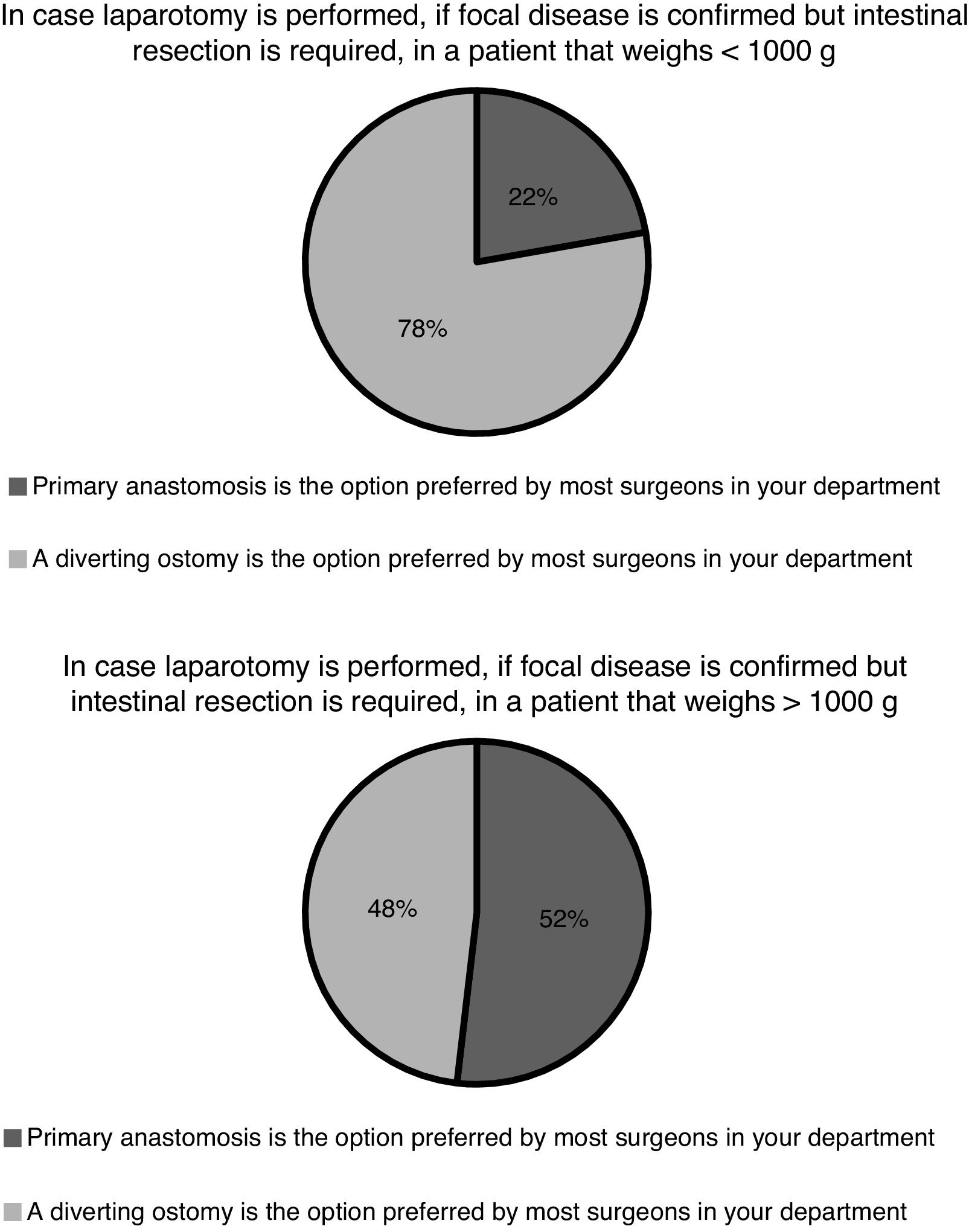
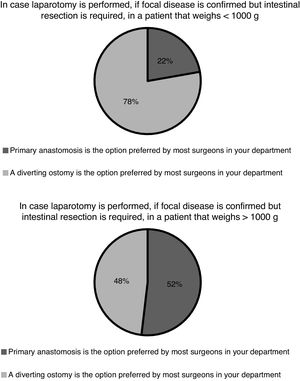
Following surgery, in the absence of complications, patients remain under complete bowel rest for at least 7 days in 20 hospitals (74%), for 7–10 days in 6 hospitals (22%) and for more than 10 days in only 1 hospital (4%). In case an enterostomy was also performed during the operation, most units (n = 20; 74%) closed the enterostomy at 6 months, and 4 (15%) before hospital discharge, independently of the age of the patient. Ninety-three percent of the surgeons (n = 25) reported that a contrast study was routinely performed in their hospitals to rule out stenosis before the enterostomy reversal procedure. Only 1 hospital reported performing the contrast study rarely or in select patients, while 1 other did not answer this question. In any case, an enterostomy is not always performed along with intestinal resection. In case of focal disease, the practice of primary anastomosis varies between hospitals and based on the weight of the patient (Fig. 3).
DiscussionIn agreement with other surveys conducted in other developed countries,4–6 our survey evinced considerable heterogeneity in preventive, diagnostic and therapeutic practices, which largely reflects the scarcity of the current evidence from clinical trials or large observational studies.
The pathophysiology of NEC is complex, which has probably hindered its prevention and eradication. In addition to preterm birth, abnormal development of the intestinal microbiota (dysbiosis)8 and enteral hypoxia-ischaemia are among the main risk factors for enterocolitis.9,10 Breastfeeding is a clear protective factor against NEC, while the use of donor human milk has been shown to reduce risk compared to the alternative of using formula.11 In addition to the type of milk, the timing of its introduction and of the escalation until the target volume is achieved are also important, as the implementation of standardised enteral nutrition guidelines has been proven to reduce the incidence of NEC.12 The administration of probiotics also seems to reduce the risk of NEC by up to 40%–50%.13,14 On the other hand, prolonged antibiotherapy after birth15 and administration of antacids interfere with the normal development of the intestinal microbiota16 and are associated with an increased risk of NEC.17,18When it comes to enteral hypoxia-ischaemia, measures such as delayed cord clamping or umbilical cord milking could be protective, although the evidence on the subject is limited, particularly for the latter intervention.19–21
When it comes to the prevention of NEC, our findings reflect a widespread availability of banked donor human milk and implementation of breastfeeding promotion in Spain. Another positive finding was the limited duration of initial empirical antibiotherapy (48 h) in case of negative blood culture results. On the other hand, the implementation of delayed cord clamping is an area that could be improved (this was not performed in 48% of hospitals) and umbilical cord milking is a potential area for future study (implementation was low, but there is also limited evidence on its efficacy and safety22). The use of probiotics was also infrequent, although on the other hand, some of the units in the study are actively conducting research on different strains through trials that may resolve one of the most important uncertainties in the use of probiotics: the strains that would be optimal in the management of preterm infants.23
The initial diagnosis based on clinical data is very nonspecific24 and frequently requires confirmation by imaging tests. In recent years there has been a growing interest in the use of abdominal ultrasound.25 This technique allows investigation of at least 15 parameters associated with NEC at the intestinal, peritoneal or hepatic levels, but its usefulness for the purpose of clinical decision-making remains to be determined,26 and therefore its indications and use vary widely between units in Spain as well as internationally.27 Near infrared spectroscopy allows monitoring of regional oxygen saturation and calculation of tissue oxygen extraction, which could be useful to identify patients at risk of enterocolitis and to monitor clinical changes in patients following diagnosis. Still, the evidence on the use of NIRS is scarce.28 Based on the findings of our survey, most neonatal units use the abdominal ultrasound scan in the diagnostic evaluation of patients with suspected enterocolitis as the initial imaging test (45% of respondents) or, more frequently, as a second test in case of uncertainty. Its use by surgeons seems to be more widespread as part of the routine preoperative evaluation (63%). The abdominal ultrasound scan is probably more sensitive than the plain radiograph for detection of intestinal necrosis, which is the key pathological feature of the disease, but further studies are required to establish its value in prognosis and therefore in guiding treatment. When it comes to NIRS monitoring of splanchnic oxygenation, there is some clinical experience in Spain, as its use is relatively frequent based on our findings (26% of respondents reported routine use), probably derived from the longer experience with the technique accrued in higher-level units for monitoring of regional cerebral oxygen saturation.
Medical treatment is based on decompression and bowel rest, broad-spectrum antibiotherapy and life support measures (fluid replacement, transfusion of blood products, mechanical ventilation and vasoactive drugs).1 Novel therapeutic approaches have not been introduced in the past few decades. The responses to the survey evince the lack of guidelines on the optimal medical management of NEC, although there seems to be a fair amount of agreement on the duration of antibiotherapy and bowel rest. The optimal antibiotic selection, optimal duration of antibiotherapy, when anaerobic coverage is indicated, the most effective approach to decompression and the adequate duration of complete enteral fasting are some of the aspects that have not been clearly established through studies specifically designed for the purpose.
According to data from the SEN1500 network (2013–2017), 61%–69% of patients with NEC received some form of surgical care.29–33 At present, the indications for surgery and the type of surgery that should be performed are still under debate.34 Intestinal perforation is the only absolute indication for surgery. Clinical deterioration despite adequate medical treatment is a relative indication for surgery on which there is no established consensus. With the aim of providing an objective measure of deterioration, some authors have proposed the use of standardised scores to measure the level of metabolic decompensation.35 Some Spanish hospitals have integrated calculation of metabolic decompensation scores in their clinical practice, but their use is not widespread (11%) and their usefulness in the prediction of outcomes is still being investigated. Once eligibility for surgery has been established, the selection of the procedure is based on the overall clinical condition, weight and length of the patient and the number of involved intestinal segments. The role of peritoneal drainage as definitive treatment or as a bridge to laparotomy in unstable patients, the indication of primary anastomosis in patients undergoing resection or the timing of stoma reversal in case of deferred anastomosis are subjects that remain controversial. The results of trials conducted to date that compared peritoneal drainage versus laparotomy as the primary procedure have been inconclusive.36,37 On the other hand, no clinical trials have been performed comparing primary anastomosis, secondary anastomosis or different timings for secondary anastomosis, and the meta-analyses of the observational studies available in the literature have left many questions unresolved.38,39 In our survey, similar proportions of surgeons reported that management of NEC was increasingly conservative versus increasingly aggressive with surgical intervention at earlier stages. In Spain, we found the greatest difference of opinion regarding surgical treatment in relation to the role of peritoneal drainage in unstable patients, especially in the absence of pneumoperitoneum, and to the performance of primary anastomosis in patients with a weight greater than 1 kg and focal disease. The use of laparoscopy in select cases is another area under investigation, and some of our hospitals have published data on the subject.40
Some of the strengths of our survey are the high response rate and the representation of different geographical areas, which makes our findings representative of the enterocolitis throughout Spain. However, there were also limitations, such as restricting the survey to hospitals with a department of paediatric surgery, the relatively small number of questions, which did not allow us to get detailed information on certain aspects, and having limited responses to 1 representative of the department of neonatology and 1 representative of the department of paediatric surgery in each hospital. The results of the survey revealed adequate implementation of preventive measures involving promotion of breastfeeding and avoidance of formula, although ideally all Level III neonatal units should have access to human milk and breastfeeding support delivered by dedicated lactation consultants. On the other hand, the increasing implementation of delayed cord clamping and antibiotic stewardship programmes with limitation of the duration of empirical antibiotherapy could be objectives to pursue in the future with the aim of reducing the incidence of NEC nationwide. We found another opportunity for improvement in the standardization of diagnosis and treatment of NEC in our hospitals, as the survey highlighted the absence of clinical guidelines. We found less variability in the responses to items concerning areas in which there is greater consensus, reflecting adequate implementation of evidence-based practice in real-world clinical settings. Conversely, we found more variability in responses to items concerning aspects for which less evidence is available, highlighting how little is known about numerous aspects related to NEC. The role of umbilical cord milking in the prevention of enterocolitis, the optimal strains and dosage of probiotics, the usefulness of ultrasonography, NIRS and other diagnostic methods in the diagnostic and preoperative evaluations, the most suitable approach to medical treatment and the indication for surgery and the surgical strategy to be used depending on the circumstances are some of the areas requiring future research. The relatively low incidence of NEC poses challenges to clinical research and calls for performance of multicentre studies.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all the neonatologists and surgeons that submitted responses to the survey.
Please cite this article as: Zozaya C, vila-Alvarez A, Somoza Argibay I, García-Muñoz Rodrigo F, Oikonomopoulou N, Encinas JL, et al. Prevención, diagnóstico y tratamiento de la enterocolitis necrosante en recién nacidos menores de 32 semanas al nacimiento en España . An Pediatr (Barc). 2020;93:161–169.