Analgesia and sedation are a priority in paediatric intensive care. The combination of ketamine and propofol is a possible option in patients requiring prolonged or difficult sedation and to reduce the use of benzodiazepines and opiates. The aim of this study was to assess the efficacy and safety of combination ketamine and propofol in continuous infusion for prolonged analgesia/sedation in the paediatric intensive care setting.
Patients and methodsProspective, observational single-group cohort study in patients aged 1 month to 16 years admitted to the paediatric intensive care unit in 2016–2018 that received ketamine and propofol in continuous infusion for analgesia and sedation. We collected data on demographic and clinical characteristics, analgesia and sedation scores (MAPS, COMFORT-B and SOPHIA), haemodynamic parameters and adverse events.
ResultsThe study included 32 patients. The maximum dose of ketamine was 1.5 mg/kg/h (interquartile range [IQR], 1−2 mg/kg/h) and the infusion duration was 5 days (IQR, 3–5 days). The maximum dose of propofol was 3.2 mg/kg/h (IQR, 2.5–3.6 mg/kg/h) and the infusion duration, 5 days (IQR, 3–5 days). Thirty (93.7%) patients had previously received midazolam and 29 (90.6%) fentanyl. Analgesia scores did not change after initiation of the ketamine and propofol infusion. There was a statistically significant increase in the COMFORT-B score, but the score remained in the adequate sedation range (12–17). There were small but statistically significant decreases in the mean arterial pressure (from 64 mmHg to 60 mmHg; P = .006) and the diastolic blood pressure (from 50.5 to 48 mmHg; P = .023) 1 h after the initiation of the ketamine and propofol infusion, but this difference was not observed 12 h later and did not require administration of vasoactive drugs. No other major adverse events were detected during the infusion.
ConclusionsThe combination of ketamine and propofol in continuous infusion is a safe treatment in critically ill children that makes it possible to achieve an appropriate level of analgesia and sedation without relevant haemodynamic repercussions.
La analgosedación es una prioridad en el cuidado de pacientes en unidades de intensivos pediátricos. La combinación de ketamina y propofol puede ser una alternativa para aquellos pacientes con necesidad de sedación prolongada, con dificultad para la sedación y para disminuir el empleo benzodiacepinas y opiáceos. El objetivo de este estudio es analizar la eficacia y seguridad de la combinación de ketamina y propofol en perfusión continua para la analgosedación en unidades de cuidados intensivos pediátricos (UCIP).
Materiales y métodosEstudio de cohorte única prospectivo observacional en pacientes de 1 mes a 16 años ingresados en UCIP entre 2016 y 2018 que recibieron tratamiento con ketamina y propofol en perfusión continua para analgosedación. Se recogieron datos clínicos y demográficos, scores de analgesia y sedación (MAPS, COMFORT-B y SOPHIA), parámetros hemodinámicos y efectos adversos.
Resultados32 pacientes fueron incluidos. La dosis máxima de ketamina fue de 1,5 mg/kg/h (RI 1−2 mg/kg/h) y la duración, 5 días (RI 3–5 días). La dosis máxima de propofol fue de 3,2 mg/kg/hora (RI 2,5−3,6 mg/kg/hora) y la duración, 5 días (RI 3–5 días). 30 pacientes (93,7%) habían recibido midazolam y 29 (90,6%) fentanilo previamente. Tras el inicio de la perfusión de ketamina y propofol la puntuación en la escala de analgesia no se modificó. El COMFORT-B mostró un incremento estadísticamente significativo, pero se mantuvo dentro del rango de sedación adecuada (12–17). Se produjo una leve disminución en la presión arterial media tras una hora de administración, que fue estadísticamente significativa (de 64 mmHg a 60 mmHg; P = 0.006) así como en la presión arterial diastólica (de 50.5 a 48 mmHg; P = 0.023). Esta diferencia desapareció a las 12 horas del inicio y no requirió uso de drogas vasoactivas. No se detectaron efectos adversos graves durante la administración.
ConclusionesLa combinación de ketamina y propofol en perfusión continua es un tratamiento seguro en cuidados intensivos pediátricos que permite un adecuado nivel de analgosedación sin repercusión hemodinámica significativa.
Ketamine acts as an antagonist of N-methyl d-aspartate (NMDA) receptors in the central nervous system, thereby inducing a state of dissociative anaesthesia. At low doses, it has an analgesic and antihyperalgesic effect. Another of its properties is an affinity for opioid receptors. Although this does not produce an analgesic effect, it may have to do with its usefulness in reducing the need for opioids and preventing withdrawal syndrome1,2. Studies on the use of ketamine for surgical procedures have demonstrated a favourable haemodynamic profile, with minimal cardiovascular depression, which is an advantage in haemodynamically unstable patients, for example after cardiac surgery3–7. However, studies on the efficacy and safety of prolonged ketamine use are needed, as those available in the current literature are mostly retrospective and conducted in small samples of patients8–11. Other groups of patients, such as critically ill neurologic patients, could also benefit, as, while it has been hypothesised that ketamine can raise intracranial pressure, there is also recent evidence suggesting that it may have a beneficial effect in these patients by decreasing intracranial pressure12,13.
Propofol acts by modulating the neurotransmitter g-aminobutyric acid (GABA) through the binding to its receptor, producing a hypnotic and sedative effect. Since it is highly lipophilic, it has a rapid onset of action in the central nervous system, and the rapid redistribution from the central to the peripheral compartment causes a quick offset of its effects. It also has a hypotensive effect through the decrease of systemic vascular resistance and heart rate14. On account of these effects, it is not as widely used in haemodynamically unstable patients. However, due to the opposing characteristics of ketamine and propofol, their combined administration may improve the adverse event profile15,16.
The 2022 guidelines for sedation in the intensive care setting of the Society of Critical Care Medicine recommend ketamine as an adjuvant treatment in paediatric patients in whom customary drugs have achieved inadequate sedation, but continue to be more restrictive toward the use of propofol in children17. The aim of our study was to assess the efficacy and safety of the combined use of ketamine and propofol in continuous infusion as part of a standard protocol for prolonged analgesia and sedation in the PICU setting.
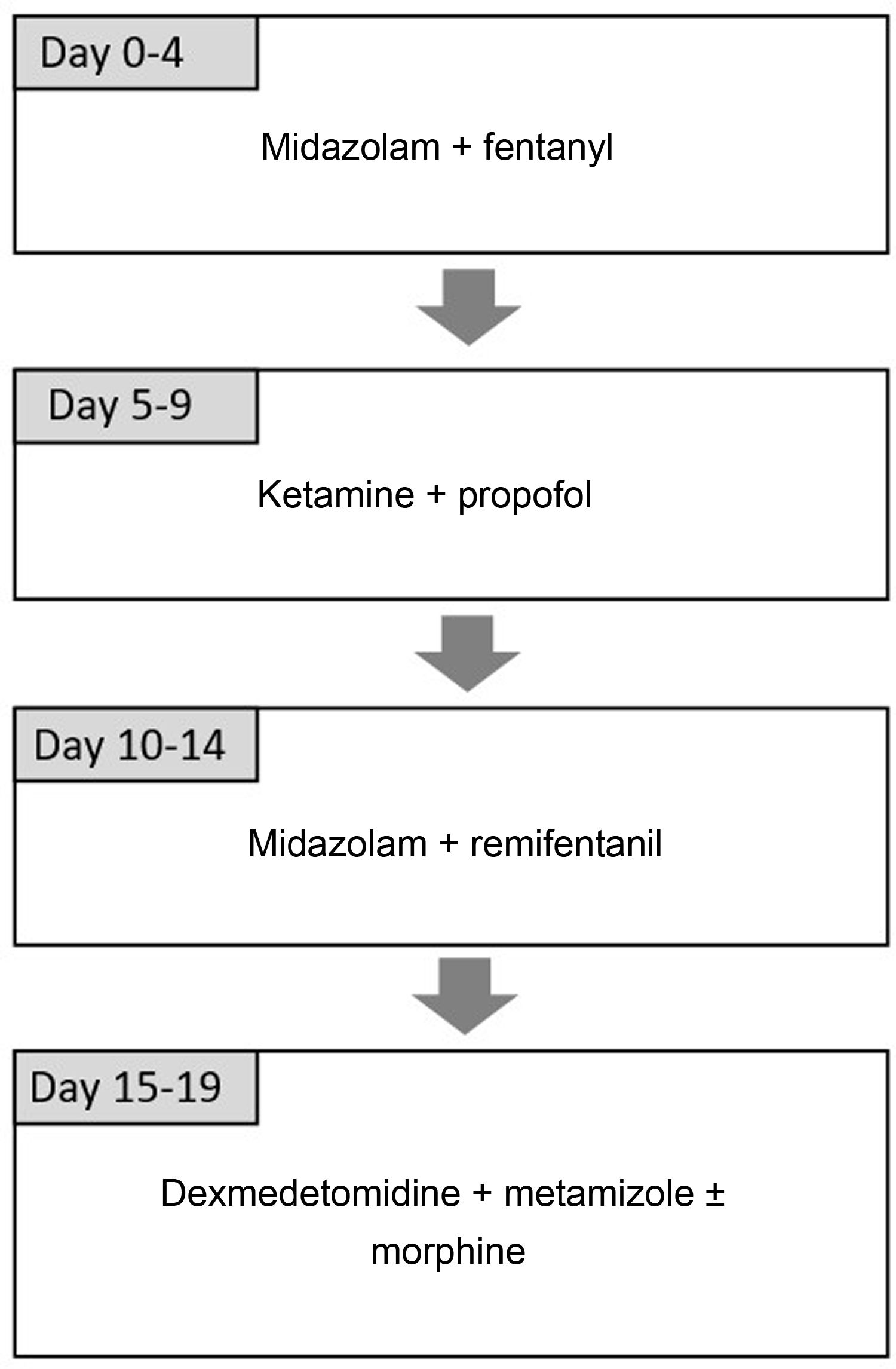
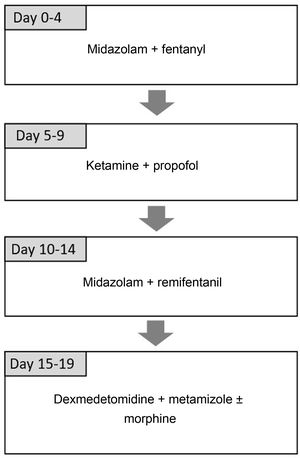
Sample and methodsWe designed a prospective observational study to assess the efficacy and safety of the combination of ketamine and propofol administered via continuous infusion in the PICU of a tertiary care hospital between September 2016 and January 2018. This combination is included in the analgesic and sedative drug rotation protocol introduced in 2012 with the aim of reducing withdrawal syndrome in critically ill patients that receive prolonged sedation18. The protocol is based on the use of different combination of analgesic and sedative drugs taking into account their pharmacokinetics and mechanisms of action, alternating opioid and non-opioid sedatives and benzodiazepine sedatives with sedatives from other groups19,20. It includes the administration of ketamine (dose of 1−2 mg/kg/h) and propofol (dose of 1−4 mg/kg/h) for 5 days. Fig. 1 presents the complete sequence. This protocol is used in every patient, although adaptations are allowed based on the judgment of the physician in charge of the patient. The study adhered to the principles of the Declaration of Helsinki of 1975, revised in 2000, and was approved by the Institutional Review Committee of our hospital under study code EVOLUCION PICU 1.2. We obtained the informed consent of the families for data collection for research purposes.
Inclusion criteriaThe study included patients aged 1 month to 16 years admitted to the PICU in the period under study that received ketamine and propofol in continuous infusion as part of the sedation and analgesia protocol. We excluded patients who received infusions of ketamine and propofol for any other reason.
Data collection and study variablesWe collected data on demographic characteristics (age and sex) and clinical characteristics, including sedation/analgesia scores, haemodynamic and laboratory parameters (lactate, triglycerides), the diagnosis at admission, the Pediatric Risk of Mortality III (PRISM III) score, previous PICU stays requiring sedation or analgesia in continuous infusion and need of mechanical ventilation, renal replacement therapy or extracorporeal circulation.
We recorded the maximum and mean doses and the duration of ketamine and propofol infusion. We collected information on the concomitant administration of analgesic and sedative drugs via continuous infusion. The nursing team documented the quality of analgesia and sedation at 3 timepoints using the following validated scales: 1) Multidimensional Assessment Pain Scale (MAPS); 2) COMFORT behaviour scale (COMFORT-B, sedation) and 3) Sophia Observation withdrawal Symptoms scale (SOS)21–24. Scores were obtained from the day before continuous infusion was initiated to up to 24 h after it ended. Adequate sedation was defined as a COMFORT-B score between 12 and 17 points and adequate analgesia as a score between 0 and 1. We defined withdrawal syndrome as a SOS score equal to or greater than 4.
We recorded haemodynamic parameters such as the systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), heart rate (HR) and the need of vasoactive drugs through the vasoactive index score (VIS) or antihypertensive treatment. This information was collected at three timepoints: 1 h before and 1 and 12 h after initiation of combined ketamine and propofol.
In addition, we documented the adverse events associated with the infusion of ketamine (hypertension, tachycardia, arrythmia, bronchorrhoea, nystagmus, agitation and delirium) and propofol (symptoms characteristic of propofol infusion syndrome, such as arrhythmia, acidosis, hypertriglyceridemia, rhabdomyolysis and hepatomegaly). Bronchorrhoea was defined as an increase in respiratory secretions and was considered significant when this increase interfered with adequate ventilation. Hypotension or hypertension requiring treatment and the development of symptoms of propofol infusion syndrome were categorised as serious adverse events.
Statistical analysisWe conducted the statistical analysis with the statistical package SPSS version 25.0 (IBM Corp; Armonk, NY, USA). Categorical variables are expressed as absolute frequencies and percentages, and continuous variables as median and interquartile range (IQR). To compare continuous variables in independent groups, we used the Kruskal Wallis test. To assess changes from before to after initiation of ketamine, we used Wilcoxon test for paired samples. Statistical significance was defined as P < .05.
ResultsThirty-two patients received ketamine and propofol via continuous infusion for more than 24 h in the period under study. The median age was 6 months (IQR, 2–48.5 months) and the median PRISM III score 8 (IQR, 3–12). Nineteen patients (59.4%) had a congenital heart defect. Table 1 presents other baseline characteristics of the sample.
Baseline characteristics of the patients.
| N = 32 | % | |
|---|---|---|
| Sex (male) | 25 | 78.1 |
| Diagnosis | ||
| Cardiac surgery | 11 | 34.3 |
| Other type of surgery | 1 | 3.1 |
| Haemodynamic | 6 | 18.8 |
| Respiratory | 13 | 40.6 |
| Infectious disease | 1 | 3.1 |
| Previous PICU stays | 16 | 50.0 |
| ECMO | 5 | 15.6 |
| Renal replacement therapy | 4 | 12.5 |
ECMO, extracorporeal membrane oxygenation; PICU, paediatric intensive care unit.
Thirty patients (93.7%) received midazolam and 29 (90.6%) fentanyl in the 5 days preceding the introduction of ketamine and propofol. The median dose of midazolam was 2 μg/kg/minute (IQR, 2–3.2 μg/kg/minute; maximum, 6 μg/kg/minute), while the median dose of fentanyl was 2 μg/kg/h (IQR, 1−2.2 μg/kg/h; maximum, 5 μg/kg/h). The median dose of ketamine at treatment initiation was 1 mg/kg/h (IQR, 1–1.5 mg/kg/h). The median maximum dose for continuous infusion was 1.5 mg/kg/h (IQR, 1−2 mg/kg/h) and the median overall maximum dose (including boluses) was 1.6 mg/kg/h (1.2−2 mg/kg/h). The highest dose was 2 mg/kg/h and was used in 43.8% of cases. The median duration of infusion was 5 days (IQR, 3–5 days). The maximum duration was 8 days. The median duration of ketamine discontinuation was 2 h (IQR, 1−5 h), with a maximum of 24 h. There were no significant differences in the maximum dose of ketamine based on age (infants, 1.5 mg/kg/h vs age > 12 months, 1.5 mg/kg/h; P = .785), or between patients previously admitted to the PICU versus patients without a previous PICU stay (1.5 mg/kg/h vs 1.5 mg/kg/h; P = .468).
Per the hospital analgesia and sedation protocol, 32 patients (100%) received propofol in continuous infusion in combination with ketamine. The median maximum dose of propofol (including continuous infusion and boluses) was 3.2 mg/kg/h (IQR, 2.5–3.6 mg/kg/h). In 3 patients, propofol was switched to midazolam and in 1 by dexmedetomidine. One patient received midazolam and ketamine simultaneously. Twenty-four patients (74.2%) did not need concomitant pharmacotherapy during the treatment with ketamine and propofol. Eight (25.8%) received other treatments at the same time as intravenous ketamine and propofol for more than 24 h. In 2 patients, these concomitant treatments were already being administered at the time of treatment with fentanyl and midazolam before initiation of continuous infusion of ketamine and propofol, and were not suspended at that point. The other 6 patients (19%) required the introduction of adjuvant drugs after the initiation of continuous infusion of ketamine and propofol. These adjuvant treatments were: continuous infusion of metamizole (n = 3; 9%), midazolam (n = 1; 3%), dexmedetomidine (n = 4; 12.5%), continuous infusion of morphine (n = 1; 3%), inhaled sevoflurane (n = 1; 3%), fentanyl (n = 1; 3%) and remifentanil (n = 1; 3%). For metamizole, the maximum dose was 6.6 mg/kg/h, while for dexmedetomidine it was 1.2 μg/kg/h. The maximum dose of morphine was 20 μg/kg/h and the maximum dose of remifentanil 12 μg/kg/h.
The median scores in the MAPS, COMFORT-B and SOS scales prior to the introduction of combined ketamine and propofol and in the 5 days following treatment initiation can be found in the Supplemental material. There were no significant differences in the median scores in the MAPS and SOS before and after initiation of continuous infusion (Days 1−5) (MAPS 0–2 and SOS < 4) (Appendix B, Supplemental material). The level of analgesia did not vary with the introduction of ketamine and propofol (score: 1). The COMFORT-B score increased significantly comparing days 1–5 of treatment to the day before initiation of ketamine and propofol, but remained within 12–17 points (Supplemental material). In one patient, propofol and ketamine were discontinued because they did not achieve an adequate level of analgesia and sedation, switching to remifentanil and dexmedetomidine. Four patients had a SOS score greater than 4 in the first 48 h after discontinuation of ketamine and propofol (12.9%).
Table 2 presents the haemodynamic parameters measured during the administration of ketamine and propofol in continuous infusion. We did not find significant differences in the VIS before and after initiation of ketamine and propofol (P = .888). We found statistically significant differences between the MAP at baseline and at 1 h of infusion (64 mmHg compared to 60 mmHg; P = .023) and in the DBP at baseline and after 1 h of ketamine and propofol (50.5 compared to 48 mmHg; P = .023). We did not find differences between baseline and 12 h after initiation of ketamine and propofol (Table 2). One patient required noradrenaline 12 h after initiation of ketamine and propofol. None of the patients required antihypertensive drugs.
Haemodynamic parameters before and after initiation of ketamine and propofol infusion.
| Before infusion | At 1 h of infusion | P | At 12 h of infusion | P | |
|---|---|---|---|---|---|
| VISn = 32 | 11.5 (0−16.5) | 11.5 (0−16.5) | .66 | 11.5 (0−16.5) | .89 |
| SBP (mmHg)n = 29 | 85 (72−99) | 84 (71−98.5) | .35 | 87.5 (73.3−98.8) | .46 |
| MAP (mmHg)n = 30 | 63 (56.8−71) | 59.5 (52.8−68) | .01* | 63 (54.5−71) | .49 |
| DBP (mmHg)n = 29 | 50 (45.5−58) | 48 (43.5−54) | .04* | 49 (43.3−58.5) | .49 |
| HR (bpm)n = 30 | 130 (110−146.3) | 130 (108.8−140) | .85 | 125 (112.5−152.5) | .14 |
Data expressed as median and interquartile range.
bpm, beats per minute; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure; VIS, vasoactive index score.
We did not find any differences in haemodynamic parameters or changes in the VIS in the 5 patients undergoing extracorporeal membrane oxygenation (ECMO) (Table 3).
Haemodynamic parameters in patients managed with extracorporeal circulation before and after initiation of ketamine and propofol infusion.
| N = 5 | Before infusion | At 1 h of infusion | P | At 12 h of infusion | P |
|---|---|---|---|---|---|
| VIS | 17 (9−38) | 17 (9−38) | 1.00 | 15 (9−33) | .18 |
| SBP (mmHg) | 86 (75.5−114.5) | 84 (67−115) | .69 | 82 (61.5−127.5) | .89 |
| MAP (mmHg) | 65 (63−74) | 66 (56−70.5) | .28 | 69 (53−79) | .69 |
| DBP (mmHg) | 58 (49−64) | 54 (48−60) | .42 | 50 (48.5−68.5) | .89 |
| HR (bpm) | 130 (117.5−162.5) | 140 (122.5−140) | .58 | 135 (117.5−137.5) | .27 |
Data expressed as median and interquartile range.
bpm, beats per minute; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure; VIS, vasoactive index score.
The most frequent adverse event during ketamine and propofol infusion was bronchorrhoea (n = 12; 37.5%), although it was not clinically significant in most cases (n = 11; 91.6%). Three patients developed agitation. In one of them, both propofol and ketamine were discontinued and treatment with midazolam and fentanyl resumed, since propofol and ketamine are not effective for controlling agitation. An opioid infusion was initiated in the other 2 patients (morphine and remifentanil). There were no cases of nystagmus or arrythmia. We did not find an association between the maximum dose or the duration of ketamine and propofol infusion and the development of adverse events. The combination of ketamine and propofol was associated with a significant increase in triglyceride levels (from 127 mg/dL to 206 mg/dL; P < .001). There was also a mild increase in lactate levels (from 1 mg/dL to 1.8 mg/dL; P < .001). None of the patients developed rhabdomyolysis or hepatomegaly. There were no deaths during infusion of ketamine and propofol.
DiscussionWe present one of the few studies describing the prolonged use of ketamine and propofol administered in combination as continuous infusion in a PICU. Our study contributes evidence on the usefulness of combined ketamine and propofol for maintenance analgesia/sedation and included patients managed with ECMO and renal replacement therapy.
The doses of ketamine and propofol used in our unit were similar to those administered in previous studies8–11,15,25. The duration of ketamine infusion in previous studies ranged from 2 to 6 days, and it was of 5 days in our study1,2,8,26.
The combination of ketamine and propofol achieved an analgesic and sedative effect comparable to those achieved with previous treatments (fentanyl and midazolam). We considered the analgesic effect, assessed by means of validated scales, adequate (MAPS < 2). Although the score in the COMFORT-B scale increased with the introduction of the combination of drugs, the sedative effect remained in the range considered adequate (12–17)21. These results are consistent with those of other studies in the PICU setting that found adequate levels of analgesia and sedation using each of these drugs separately although there is still a dearth of data on their use in combination for prolonged analgesia1,2,8,27,28. It is worth highlighting that in 81% of cases, an adequate level of analgesia and sedation was achieved without needing to add any adjuvant drugs. The remaining 19% of patients received some other intravenous drug in continuous infusion in addition to propofol and ketamine. In these cases, it would be reasonable to consider that ketamine and propofol did not achieve the desired level of sedation. Fentanyl withdrawal may have played a significant role in these patients, as in most previous studies treatment with ketamine was initiated while maintaining the initial opioid treatment8,10,11, whereas in our study, opioid treatment was discontinued on initiating ketamine and propofol.
In one patient, the infusion of ketamine and propofol was discontinued on account of inadequate analgesia/sedation due to agitation. Agitation has been reported as an adverse effect of ketamine in procedural sedation, although it is more frequent with other drugs, such as benzodiazepines29.
Although the evidence in the paediatric population is limited, it seems that the haemodynamic impact of ketamine results from two opposing mechanisms: a direct negative inotropic effect and an indirect positive inotropic effect induced by the increase in the concentration of endogenous catecholamines30. Propofol produces a decrease in blood pressure through a decrease in vascular capacitance, myocardial contractility and autonomic control of cardiac output15. The combination of ketamine and propofol for procedural sedation in paediatric patients has proven safe, without significant decreases in heart rate or blood pressure, but studies assessing its effectiveness and safety for prolonged sedation are still lacking15,31,32. Few studies to date have included patients with congenital heart disease. It is important to consider potential haemodynamic changes when assessing the effects of ketamine and propofol in continuous infusion. A study on analgesia and sedation in paediatric patients undergoing cardiac catheterization found that the combination of ketamine and propofol reduced the cumulative dose of propofol and preserved the mean arterial pressure16.
We found a small but statistically significant decrease in the MAP and DBP in the first hour of ketamine and propofol infusion that normalised afterwards without requiring increased vasoactive drug support or fluid replacement therapy. This suggests that the use of combined ketamine and propofol in continuous infusion does not have significant haemodynamic repercussions, even in patients with cardiac disease.
In this study, none of the patients had serious adverse events associated with the administration of ketamine and propofol. Bronchorrhoea developed in more than one third of patients, an effect previously described in relation to ketamine, but it did not have a significant impact in respiratory function and did not require treatment with anticholinergic drugs. Other studies have had similar findings8,10,26. Previous clinical case series have reported propofol infusion syndrome in paediatric patients, chiefly with the use of doses much greater than 4 mg/kg/h. Propofol infusion syndrome is characterised by bradycardia, metabolic acidosis, hypertriglyceridemia, rhabdomyolysis and hepatomegaly33. None of the patients developed bradycardia, rhabdomyolysis or hepatomegaly, and the increases in the levels of triglycerides and lactate, while statistically significant, were not clinically relevant.
We did not detect other adverse effects described in the literature (intracranial or ocular hypertension). This limits the extrapolation of our findings to patients at risk of developing these adverse effects34–36.
Four patients (12.5%) developed withdrawal symptoms after discontinuation of ketamine and propofol. However, this must be interpreted with caution, given the concomitant use of other pharmaceuticals.
One of the limitations of our study was the small number of included patients. Still, it is one of the few studies with a prospective design, and the sample was larger compared to previous publications. It was an observational study that documented real-world analgesia and sedation practices in in our hospital. The observational design and lack of a control group are limitations that must be taken into account in the interpretation of the results.
It is also important to consider the use of other sedatives and analgesics in interpreting our findings. However, this is an unavoidable limitation, as in clinical practice adjuvant drugs are widely used in the sedation of critically ill children. Another limitation was the lack of data on the administration of analgesic and sedative drugs via the enteral route.
ConclusionThe combination of ketamine and propofol delivered through continuous infusion may be an appropriate treatment for paediatric patients admitted to intensive care units, as it achieves adequate levels of analgesia and sedation and allows discontinuation of opioids and benzodiazepines. Ketamine and propofol have a good safety profile and do not cause significant haemodynamic effects or adverse events. However, it is essential to obtain further data through prospective controlled trials conducted in larger samples.
FundingThis study did not receive specific financial support from any public, private or not-for-profit institutions.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the team of doctors and nurses of the intensive care unit for their participation in care delivery and their collaboration in data collection and documentation.
Meeting presentation: partial results of this study were presented as a brief communication at the XXXIII Annual Congress of the Sociedad Española de Cuidados Intensivos Pediátricos; June 10–13, 2018; Granada, Spain.