Reflux nephropathy is a radiologic condition commonly used to express the existence of renal morphological lesions in patients who have or had vesicoureteral reflux (VUR). This morphological concept is used based on the image data collected, without conducting basic complementary renal function studies. The present study was designed to demonstrate that patients with active VUR present different functional renal alterations from those shown by patients with disappeared VUR.
MethodsLongitudinal descriptive retrospective analysis including 89 children (46M, 43F) with VUR diagnosis through a standard voiding cystourethrogram (VCUG). The basic renal function tests collected were the maximum urinary osmolality (UOsm) and the urinary albumin/creatinine and NAG/creatinine ratios. The data collected corresponded to two moments, when VUR was diagnosed and when it had already disappeared.
ResultsQuantitative differences were verified in the three functional parameters when comparing those corresponding to both moments of the study. In the qualitative analysis, in relation to the intensity of the VUR, differences were observed in UOsm at diagnosis and in the albumin/creatinine ratio once the VUR had cured. At this last moment, a significant increase in the albumin/creatinine ratio was observed in patients with loss of renal parenchyma in relation to those without residual morphological lesions.
ConclusionsConcentrating ability defect is the most frequent finding in children with active reflux (true reflux nephropathy), whereas the most frequent functional disturbance found, once VUR has cured, is an increase in urinary albumin excretion, related to parenchymal damage. The term dysplastic-scarring nephropathy, could be more appropriate for patients with residual morphological lesions and impaired renal function, once VUR is cured.
La nefropatía de reflujo es el término radiológico que se utilizó para expresar la existencia de lesiones morfológicas renales en pacientes con reflujo vesicoureteral (RVU). Este concepto morfológico se acuñó a partir de los datos de imagen recogidos aunque sin realizar estudios complementarios básicos de función renal. Este estudio se diseñó para demostrar que las pruebas de función renal básicas muestran resultados distintos en presencia de RVU activo y una vez desaparecido.
Pacientes y métodosEstudio descriptivo retrospectivo longitudinal en el que se incluyeron 89 niños (46V, 43M) con RVU diagnosticado mediante cistouretrografía miccional seriada. Las pruebas básicas de función renal recogidas fueron la osmolalidad urinaria máxima (UOsm) y los cocientes urinarios albumina/creatinina y NAG/creatinina. Los datos acopiados correspondían a dos momentos, al diagnosticarse el RVU y cuando ya se había curado.
ResultadosSe comprobaron diferencias cuantitativas en los tres parámetros funcionales al comparar los correspondientes a ambos momentos del estudio. En el análisis cualitativo, en relación con la intensidad del RVU, se apreciaron diferencias en UOsm al diagnóstico y en el cociente albumina/creatinina una vez desaparecido el RVU. En este último momento, se observó un aumento significativo en el cociente albumina/creatinina en los pacientes con pérdida de parénquima renal en relación con aquellos sin lesiones morfológicas residuales.
ConclusionesEl defecto en la capacidad de concentración renal es el hallazgo más frecuente en niños con RVU activo (auténtica nefropatía de reflujo), mientras que una vez curado el RVU, la alteración funcional más frecuente es el aumento de la excreción de albúmina, en relación con el daño residual existente en el parénquima renal. Puesto que la alteración funcional es diferente en los niños con RVU activo y cuando ya no está presente, no se debería utilizar el mismo término para ambas situaciones puesto que, aunque próximas, son entidades distintas. El término nefropatía displásico-cicatrizal reflejaría mejor las características de estos pacientes una vez curado el RVU.
Chronic pyelonephritis was the first term coined to describe permanent residual renal lesions observed in children with a history of one or more febrile urinary tract infections (UTIs).1 In the 1960s, evidence emerged of a potential association between chronic pyelonephritis and vesicoureteral reflux (VUR), which motivated the diffusion of a new term, reflux nephropathy.2 This new concept, initially based on radiological findings, referred to an association between VUR, renal scarring and the possibility of developing chronic renal disease,3 although little attention was given to the presence of other associated renal functional defects. In the late 1990s, avoiding this term was recommended, as it was established that sterile VUR did not cause scarring,4 and that infection of the renal parenchyma, rather than VUR, was the necessary condition for its development,5 which means that VUR is not the actual cause of the scarring, but rather the acute inflammation of the renal parenchyma.4–7 Thus, acquired renal scarring was proposed as an alternative to replace the term reflux nephropathy.8 However, the new term underemphasised congenital defects (dysplasia, hypodysplasia) that may be associated with VUR and that have come to be known as congenital reflux nephropathy.8,9
Preliminary studies have shown that VUR interferes with renal tubule function. Thus, a reduction in renal concentrating ability10,11 and an increase in the urinary excretion of albumin12 and of N-acetyl-β-glucosaminidase (NAG)13 have been reported.
We designed a study to assess whether the abnormalities observed in basic renal function tests were different when VUR was active versus, once VUR had resolved, only the residual congenital or acquired loss of renal parenchyma remained.
Sample and methodsStudy designWe conducted a longitudinal retrospective and descriptive study including data corresponding to 89 patient aged less than 16 years (46 male, 43 female) with a diagnosis of VUR made by voiding cystourethrogram (VCUG) between January 2003 and December 2016. The patients had been followed up for a minimum of 2 years at the outpatient paediatric nephrology clinic of our hospital with at least the maximum urine osmolality (UOsm) documented in their health records by the end of the followup. OF these patients, 62 had undergone surgery and 27 had received conservative treatment.
Th mean age at diagnosis was 23.9 months (standard deviation, 31.1; range, 0–164 months). Thirty-nine children received the diagnosis of VUR before age 6 months and 50 after. Seventy-one patients were evaluated for having more than 1 UTI. Another 16 underwent evaluation after detection of structural abnormalities in antenatal ultrasound examinations (hydronephrosis in 13 and small kidneys in 3). Macroscopic haematuria was the initial warning sign in the 2 remaining patients.
Vesicoureteral reflux was categorised into 5 grades (I–V) based on the classification of the International Reflux Study Committee.14 The highest grading was assigned to cases of bilateral VUR (n=43). Seventeen cases were classified as mild VUR (2 grade I, 15 grade II), 39 as moderate (grade III) and 33 as severe (27 grade IV, 6 grade V).
Vesicoureteral reflux was managed conservatively (with or without antibiotic prophylaxis) in 27 patients (30.3%), with open surgery in 15 cases (16.8%) and with endoscopic surgery in 47 (52.8%). Vesicoureteral reflux was resolved at a mean age of 5.86 years (SD, 3.65; range, 1.06–14.83). All patients were aged more than 2 years by the end of the followup (mean, 6.28 years; SD, 3.75; range, 2–16).
For the diagnosis timepoint, we used the data for samples obtained at the time closest to the VCUG. Samples for the determination of the renal function parameters under study were obtained at least 4 months after resolution of an acute pyelonephritis episode.
Inclusion criteriaThe inclusion criteria were the following:
- a)
Primary VUR diagnosed by VCUG that resolved with conservative or surgical management.
- b)
Performance of at least 1 imaging test (ultrasound or a renal scan with 99m-technetium dimercaptosuccinic acid [99mTc-DMSA]) at each of the 2 timepoints under study (at diagnosis and at the end of followup).
- c)
Documentation in the health records of at least the la UOsm at the end of followup. We also collected data on other renal function parameters (albumin/creatinine and NAG/creatinine ratio) when available.
We excluded all patients aged more than 16 years with VUR and all children of any age with a diagnosis of multicystic renal dysplasia, pyeloureteral or vesicoureteral stenosis, posterior urethral valves or neurogenic bladder.
Assessment of renal structureAn ultrasound was performed at diagnosis in 85 patients and in every patient by the end of the followup (n=89). Mild hydronephrosis was diagnosed in children with an anteroposterior renal pelvis diameter of 0.5–2cm, and moderate/severe hydronephrosis if the diameter was 2cm or greater. A 99mTc-DMSA renal scan was performed in 72 patients at diagnosis and in 80 at the end of the followup. Congenital renal dysplasia was diagnosed based on detection of small-sized kidneys with structural abnormalities and a relative tracer uptake of less than 20% in the initial renal scan.15
Desmopressin challenge testAfter voiding the bladder, the patient was given 20μg of desmopressin intranasally, 0.2mg (200μg) of desmopressin in tablet form, or else 0.12mg (120μg) of oral lyophilisate (DDAVP MELT) that dissolves instantly in the oral cavity. After the administration of desmopressin, we collected 3 consecutive urine samples, at 90-minute intervals if the child had achieved bladder control. We imposed moderate restrictions on fluid intake. In the case of infants aged 1–12 months, we administered a 10μg dose of desmopressin intranasally in the morning and restricted oral feeds to half the normal volume until 6pm to decrease the risk of water intoxication. The maximum UOsm was recorded as the result of the challenge.11,16
Laboratory testsUrine creatinine was determined with a kinetic colorimetric assay based on the Jaffe method (Creatinine Jaffe Gen.2, Roche). Albumin was measured with a rate nephelometry method (array) and NAG activity using a colorimetry assay based on the hydrolysis of 3-cresolsulfonphthaleinyl-N-acetyl -β-d-glucosaminidase (Roche). The UOsm was measured with freezing point depression osmometer (Osmo Station OM-6050, Menarini Diagnostics).
Normal rangesThe normal ranges used as reference for the maximum UOsm following administration of desmopressin are available in a previous publication by our research group based on measurements in 125 healthy children.11 In infants, the fifth percentile (P5) is 532mOsm/kg for age 1–3 months, 616mOsm/kg for age 3–6 months; 645mOsm/kg for age 6–9 months and 740mOsm/kg for age 9–12 months. In children aged more than 1 year, the lower limit of normal is 800mOsm/kg.16 Normal ranges for age for the albumin/creatinine ratio and the NAG/creatinine ratio have also been published in the past.17
Statistical analysisWe used the Kolmogorov-Smirnov test to analyse the distribution of the data. In the descriptive analysis, we summarised categorical data as percentages and continuous data as mean±standard deviation if they were normally distributed, and otherwise (albumin/creatinine and NAG/creatinine ratios) as median and interquartile range (IQR). We compared variables with analysis of variance (ANOVA) or the Kruskal-Wallis test based on whether they were or not normally distributed. We assessed differences between paired samples by means of the Friedman test or Wilcoxon test. We used the chi square test to compare qualitative variable frequencies in groups, using the Yates correction as applicable. We calculated parameters of diagnostic performance to compare the data for cases of severe VUR to cases of mild/moderate VUR, both when VUR was active and when it was cured. Thus, we calculated the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), the positive likelihood ratio (LR+) and negative likelihood ratio (LR–) and the odds ratio (OR) with the corresponding 95% confidence interval (CI) for the 3 markers of renal function under study. The analysis was performed with the statistical software SPSS version 20.0 (SPSS Inc, Chicago, IL, USA). We defined statistical significance as a P-value of less than .05.
ResultsAt the time of diagnosis, imaging findings were abnormal in 52 children: renal dysplasia (n=14), an area of unilateral scarring (n=14), several areas of scarring (n=11), mild isolated hydronephrosis (n=7), moderate-severe isolated hydronephrosis (n=1), duplicated collecting system (n=4) and renal agenesis (n=1). In addition, in 14 children, the acute-phase 99mTc-DMSA renal scan revealed areas of reduced tracer uptake characteristic of acute pyelonephritis. Renal imaging findings were normal in the rest of cases (n=23). Of the 14 children with renal dysplasia, 10 were male, 8 had grade IV VUR, and 6 had grade III VUR.
At the end of followup, 19 patients had unilateral renal scarring (one of them had a duplicated collecting system) 16 had several areas of scarring, 14 had a dysplastic kidney, as mentioned above, and 9 renal atrophy; one patient had renal agenesis. Renal morphology was normal in the 30 remaining children (3 with a duplicated collecting system). In short, 59 patients had a decrease in renal parenchyma of varying degree and 30 had an apparently intact renal parenchyma.
Table 1 presents the qualitative analysis of the renal function parameters in the patients, grouped by the grade of VUR a baseline, at diagnosis and at the end of followup. We found statistically significant differences in the UOsm, which at the time of diagnosis was decreased in 21% of children with mild VUR, 37% of children with moderate VUR and 67% of children with severe VUR. At the end of followup, we found a statistically significant difference in the frequency of patients with an elevated albumin/creatinine ratio based on the grade of reflux: none with mild VUR, 10% of children with moderate VUR and 39% of children with severe VUR (Table 1).
Cualitative analysis of the renal function parameters in relation to the grade of VUR at the time of diagnosis and at the end of followup.
| Mild VUR | Moderate VUR | Severe VUR | P | ||||
|---|---|---|---|---|---|---|---|
| Maximum UOsm at diagnosis (active VUR) | Normal 11 | Decreased 3 | Normal 22 | Decreased 13 | Normal 10 | Decreased 20 | .008 |
| Maximum UOsm at end of followup (resolved VUR) | Normal 17 | Decreased 0 | Normal 37 | Decreased 2 | Normal 27 | Decreased 6 | ns |
| Albumin/creatinine at diagnosis (active VUR) | Normal 11 | Elevated 1 | Normal 25 | Elevated 3 | Normal 19 | Elevated 8 | ns |
| Albumin/creatinine at end of followup (resolved VUR) | Normal 16 | Elevated 0 | Normal 30 | Elevated 3 | Normal 23 | Elevated 9 | .02 |
| NAG/creatinine at diagnosis (active VUR) | Normal 7 | Elevated 0 | Normal 13 | Elevated 6 | Normal 10 | Elevated 4 | ns |
| NAG/creatinine at end of followup (resolved VUR) | Normal 8 | Elevated 0 | Normal 22 | Elevated 0 | Normal 18 | Elevated 0 | – |
NAG, N-acetyl-β-d-glucosaminidase; ns, not significant; UOsm, urinary osmolality; VUR, vesicoureteral reflux.
Using the data presented in Table 1, we analysed diagnostic performance parameters to compare the findings in severe VUR versus mild to moderate VUR in both timepoints under study (Table 2). The maximum UOsm had a sensitivity of 66.67% for discrimination of both types of VUR, which dropped to 18.18% after VUR resolved. The sensitivity of the other 2 parameters at the time of diagnosis was low. Once VUR had resolved, the specificity of both the maximum UOsm and the albumin/creatinine ratio was greater than 90%, and the specificity of the NAG/creatinine was 100%. The odds ratio was statistically significant for the maximum UOsm and urinary albumin at both timepoints (Table 2).
Results of the parameters used to analyse the diagnostic yield and accuracy of the basic renal function markers under study in severe versus mild to moderate vesicoureteral reflux at diagnosis and at the end of followup.
| Sen (95% CI) | Spe (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR– (95% CI) | Odds ratio | |
|---|---|---|---|---|---|---|---|
| P (95% CI) | |||||||
| Maximum UOsm at diagnosis (active VUR) | 66.67% (47.19−82.71) | 67.35% (52.46−80.05) | 55.56% (43.73−66.78) | 76.74% (65.74−85.02) | 2.04 (1.27−3.28) | 0.49 (0.29−0.85) | 4.12 |
| P=.004 (1.57−10.83) | |||||||
| Maximum UOsm at end of followup (resolved VUR) | 18.18% (6.98−35.46) | 96.43% (87.69−99.56) | 75.00% (39.11−93.34) | 66.67% (62.82−70.30) | 5.09 (1.09−23.78) | 0.85 (0.72−1.00) | 6 |
| P=.035 (1.13−31.7) | |||||||
| Albumin/creatinine at diagnosis (active VUR) | 29.63% (13.75−50.18) | 90.00% (76.34−97.21) | 66.67% (40.05−85.69) | 65.45% (59.23−71.19) | 2.96 (0.99−8.87) | 0.78 (0.60−1.02) | 3.78 |
| P=.048 (1.00−14.2) | |||||||
| Albumin/creatinine at end of followup (resolved VUR) | 28.12% (13.75−46.75) | 93.88% (83.13−98.72) | 75.00% (46.76−91.11) | 66.67% (61.42−71.53) | 4.59 (1.34−15.69) | 0.77 (0.61−0.96) | 6 |
| P=.01 (1.48−24.31) | |||||||
| NAG/creatinine at diagnosis (active VUR) | 28.57% (8.39−58.1) | 76.92% (56.35−91.03) | 40.00% (18.38−66.38) | 66.67% (57.46−74.76) | 1.24 (0.42−3.67) | 0.93 (0.63−1.27) | 1.33 |
| ns (0.30−5.83) | |||||||
| NAG/creatinine at end of followup (resolved VUR) | 0% (0−18.53) | 100% (88.43−100) | – | 62.5% (62.5−62.5) | – | 1 (1−1) | 1.64 |
| ns (0.03−86.68) |
CI, confidence interval; LR–, negative likelihood ratio; LR+; positive likelihood ratio; NAG, N-acetyl-β-d-glucosaminidase; NPV, negative predictive value; ns, not significant; PPV, positive predictive value; Sen, sensitivity; Spe, specificity; UOsm, urinary osmolality; VUR, vesicoureteral reflux.
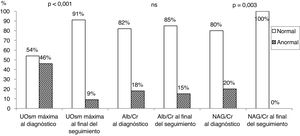
Table 3 presents the results of the quantitative and qualitative analysis of renal function parameters in the initial timepoint (at diagnosis of VUR) and at the end of followup (resolved VUR). We found significant differences in the quantitative values of the 3 parameters under study. At diagnosis, 46% of children exhibited a decreased concentrating ability, 18% an increased albumin/creatinine ratio, and 20% an increased NAG/creatinine ratio. At the end of followup, the renal concentrating ability (maximum UOsm) was decreased in only 9% of patients, and the albumin/creatinine ratio increased in 15%. The NAG/creatinine ratio was normal in every patient with resolved VUR (Fig. 1). Of the 8 patients with an impaired renal concentrating ability at the end of followup, 7 (87%) exhibited a decrease in renal parenchyma (Table 3).
Quantitative and qualitative analysis of renal function parameters at the time of diagnosis of VUR and at the end of followup.
| Diagnosis of VUR (active VUR) | End of followup (resolved VUR) | P | |||
|---|---|---|---|---|---|
| Maximum UOsm (mOsm/kg) (n=79) | 670.1±245.6 | 927.9±119.3 | < .001 | ||
| Maximum UOsm | Normal 43 (54%) | Decreased 36 (46%) | Normal 81 (91%) | Decreased 8 (9%)a | < .001 |
| Albumin/creatinine (μg/μmol) (n=66) | 1.93 (3.01) | 1.05 (1.23) | < .001 | ||
| Albumin/creatinine | Normal 55 (82%) | Elevated 12 (18%) | Normal 69 (85%) | Elevated 12 (15%) | ns |
| NAG/creatinine (U/g) (n=27) | 7.37 (14.98) | 2.48 (1.8) | < .001 | ||
| NAG/creatinine | Normal 39 (80%) | Elevated 10 (20%) | Normal 48 (100%) | Elevated 0 (–) | .003 |
NAG, N-acetyl-β-d-glucosaminidase; ns, not significant; UOsm, urinary osmolality; VUR, vesicoureteral reflux.
Bar chart representing the proportion of patients in the normal and abnormal range for the 3 analysed renal function parameters at the time of diagnosis of VUR and at the end of followup (data shown in Table 3).
Alb, albumin; Cr, creatinine; NAG, N-acetyl-β-d-glucosaminidase; ns, not significant; UOsm, urinary osmolality.
At the end of followup, when we grouped patients based on whether or not they exhibited a decrease in renal parenchyma, the frequency of patients with an increased albumin/creatinine ratio was higher in the group with decreased renal parenchyma (Table 4).
Qualitative analysis of renal function parameters in relation to the loss of renal parenchyma at the end of followup (resolved VUR).
We did not present the estimated glomerular filtration rate (eGFR) calculated with the updated Schwartz formula (2009) in any of the tables. Only 3 patients had an eGFR of less than 90mL/min/1.73m2 at the end of followup; two of them exhibited a decreased concentrating ability and an increased creatinine/albumin ratio.
DiscussionMany patients in the study were managed at a time when the approach to treatment was different from the current one. Children with VUR that had undergone renal function tests seemed to have the poorest outcomes. For this reason, the frequency of surgical management (endoscopic or open surgery) was higher compared to what is usually observed in hospitals in Spain, and the frequency of scarring at the end of followup was higher compared to longitudinal studies on the subject, which reported an incidence of new scarring of approximately 10%.18,19
As we mentioned in the introduction, chronic pyelonephritis was the first term used to describe residual lesions that appeared after one or more febrile UTIs.1 Today, it may seem difficult to understand why renal lesions found in the forensic examination of children would contain bacteria by areas of fibrosis and nephron loss; features also observed in animal models of UTI, and this could be due to the lack of effective antibiotics in the beginning and the fact that the agents could not penetrate the scar tissue.20 The persistent presence of bacteria suggests that a residual bacterial population could remain in the renal parenchyma and pelvis for a period of time after a febrile UTI and could cause recurrence in some patients.
As we already mentioned, the apparent association of chronic pyelonephritis and VUR was labelled reflux nephropathy.3 This is a radiological construct that translate to a decrease in renal parenchyma and that is confusing for several reasons. On one hand, renal development anomalies characterised by a decrease of renal parenchyma (dysplasia and hypoplasia) overlap with VUR; renal dysplasia is usually associated to massive VUR, most frequently unilateral and in male patients.8,9 Renal dysplasia is not caused by vesicoureteral reflux in isolation, but rather results from the association of 2 congenital anomalies of the kidney and urinary tract (CAKUT), which is relatively common in humans. In one of the best published editorials on the subject, Fernández Menéndez and Málaga Guerrero21 wrote that the term reflux nephropathy is misleading and that “this inadequate and confusing expression is still used today all too frequently. We will be able to completely eradicate its use once we stop being confused.” On the other hand, as we noted in the introduction, the necessary condition for development of renal scarring after a febrile UTI is inflammation itself and not the presence of VUR.4 At the end of the 1950s, Shapiro et al.6 demonstrated in an experimental study in rats without VUR that induction of pyelonephritis in the animals produced an inflammatory response that eventually led to scarring.20 In this sense, scarring is a loss of renal cortical parenchyma with secondary fibrosis resulting from the immune response to bacterial invasion, independently of the presence or absence of increased pressure in the urinary tract.22 Thus, it is well established that VUR is not the ultimate cause of scarring.4–7,20,23 However, a higher risk of renal scarring has been described in patients with VUR and urinary tract dilation.24 Knowing that VUR is not a necessary condition for scar tissue formation, it is reasonable to explore why the severity of VUR may be associated with an increased risk of scarring. A possible explanation would be that pyelonephritis in patients with VUR and urinary tract dilation may be caused by bacteria that are more virulent for yet unknown reasons.25,26
As concerns basic renal function tests, an experimental study in dogs published in the 1960s found a decreased renal concentrating ability after causing ureteral blockade.27 Later, when desmopressin became available, researchers were able to measure the maximum UOsm, which turned out to decrease in patients, both paediatric and adult, with pyeloureteral obstruction.28 Since then, a few studies have described an association between VUR and a decreased renal concentrating ability.10,11,29
In recent years, the renal mechanisms that explain the changes in water excretion in patients with increased pressure in the urinary tract have been investigated. Thus, studies have study found decreased activity in sodium transporters in the tubules,30 decreased expression of urea transporters31 and reduced aquaporin activity in the collecting duct.30 Specifically, an experimental animal model of VUR found a decreased expression of aquaporins 1 and 2.32 All these functional changes promote the development of polyuria and salt wasting.
In this regard, in our sample a decreased concentrating ability was the most frequent functional impairment in patients with active VUR (Tables 1–3; Fig. 1), and it was more pronounced in patients with high-grade VUR (Table 2). Thus, a decreased maximum UOsm as the most frequent functional abnormality in patients with what we could call true reflux nephropathy. However, once VUR had resolved, the concentrating ability (maximum UOsm) improved (Table 2) and the number of children with impaired concentrating ability decreased from 46% to 9% (Table 3; Fig. 1). Of the 8 children with persistently decreased concentrating ability, 7 had some form of detectable renal parenchymal damage (Table 3).
There is evidence that supports that an increase in urinary albumin excretion is an early sign of glomerular damage in both diseases without nephron loss (diabetic nephropathy, obesity) and with a reduction in the number of nephrons, in addition to a good predictor of future cardiovascular disease. An increase in urinary albumin excretion has been described in patients with VUR,12 but it is unclear whether this is due to the reduced renal parenchyma or to the increased pressure in the urinary tract.
In our case series, albuminuria was the most frequently observed abnormality in children with parenchymal loss once VUR had resolved (Table 3). Furthermore, patients that had more severe VUR at diagnosis had higher concentrations of albumin in urine at the end of followup (Tables 1 and 2) in association with the concomitant loss of renal parenchyma.
As for NAG, since the 1960s it is known that it originates from renal tissue,33 as it is found in the lysosomes of proximal tubule cells; when these cells are damaged, it is released to the tubular lumen, resulting in its increased urinary excretion.34 At first, it was used to assess the nephrotoxicity of aminoglycoside antibiotics, but NAG levels can also be elevated in patients with increased intratubular pressure, which is the case of urinary tract obstruction35 and VUR.13 There is debate as to whether this increase in urinary NAG excretion is due to an increased pressure or to a decreased glomerular filtration rate.12 In our sample, we found an increased urinary excretion of NAG in 20% of patients with active VUR that was probably secondary to increased pressure, as NAG levels normalised in all patients once VUR had resolved, which translates to a specificity of 100% (Tables 1–3, Fig. 1).
In short, from a functional standpoint, reflux nephropathy reflects a real process and applies when VUR is active, mainly translating into a decreased renal concentrating ability. On the other hand, albuminuria is the most frequent abnormality present in acquired renal scarring (once VUR has resolved), which is associated with the residual parenchymal damage. Although these 2 renal diseases are closely related, they should not share a name because they cause different functional abnormalities in different morphological contexts, that is, when VUR is active and when it is cured. Lastly, we propose the term dysplastic-scarring nephropathy as a more fitting alternative to acquired renal scarring in patients with cured VUR, loss of renal parenchyma and impaired renal function.
In addition to its retrospective design, one of the limitations of this study was that morphological evaluations and/or renal function tests were not carried out in some of the patients at both timepoints.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García Nieto VM, Monge Zamorano M, Antón Hernández L, Luis Yanes MI, Tejera Carreño P, Moraleda Mesa T. Nefropatía de reflujo y nefropatía cicatrizal. Dos entidades tan cercanas pero funcionalmente tan distintas. An Pediatr (Barc). 2022;97:40–47.