Human metapneumovirus (Metapneumovirus hominis [hMPV]) belongs to the same family as respiratory syncytial virus (Pneumoviridae). It was identified in 2001, and there is growing awareness of its importance as a cause of acute respiratory illness. Infections by hMPV occur in winter outbreaks and may be asymptomatic or manifest in the form of bronchiolitis or pneumonia, with symptomatic presentations being most common in young children.
The COVID-19 pandemic and the hygiene measures implemented in its wake shifted clinical and epidemiological trends in respiratory infections.1 The aim of our study was to describe the clinical and epidemiological characteristics of hMPV infections in children and determine whether there were any changes in them following the COVID-19 pandemic.
We conducted a retrospective review of the health records of patients aged 21 days to 14 years admitted between January 2018 and August 2024 to a tertiary care hospital due to acute respiratory infection who had a positive PCR result for hMPV. In our hospital, a PCR multiplex assay (17 viruses) is performed routinely in any child admitted for respiratory illness. We summarized the data for epidemiological and clinical variables as percentages or median and interquartile range. We assessed differences between the prepandemic (up to March 2020) and postpandemic periods with the χ2 test or Mann–Whitney U test. The study was approved by the ethics committee of the hospital (PI-24-679H).
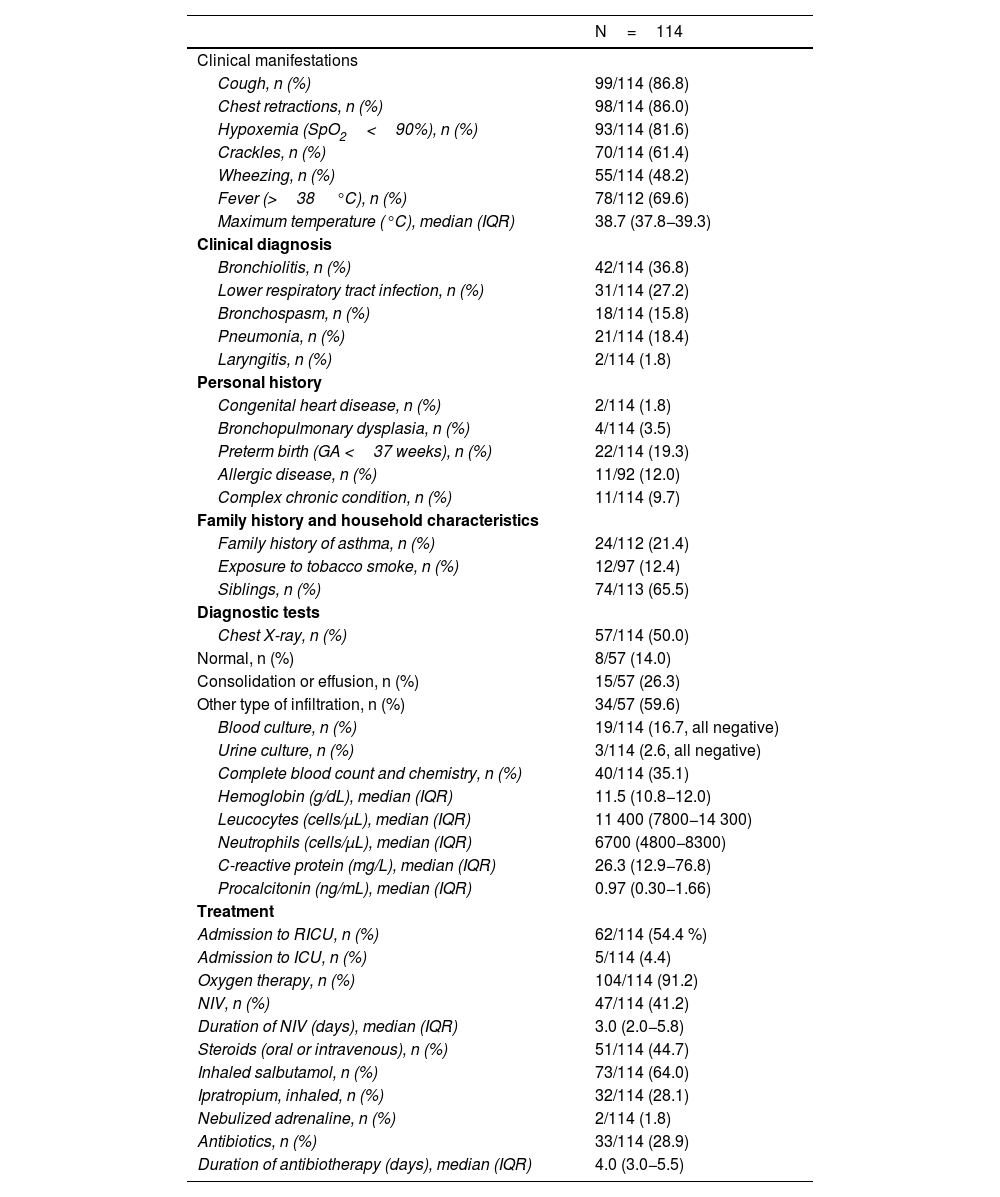
The sample included 114 admissions (52.6% of male patients) at a median age of 13.1 months (IQR, 5.3–23.4). Of this total, 9.7% corresponded to children with complex chronic conditions. The median length of stay was 4.0 days (IQR, 2.8–7.0). Table 1 presents the clinical and epidemiological characteristics of the sample.
Clinical and epidemiological characteristics of metapneumovirus infections.
| N=114 | |
|---|---|
| Clinical manifestations | |
| Cough, n (%) | 99/114 (86.8) |
| Chest retractions, n (%) | 98/114 (86.0) |
| Hypoxemia (SpO2<90%), n (%) | 93/114 (81.6) |
| Crackles, n (%) | 70/114 (61.4) |
| Wheezing, n (%) | 55/114 (48.2) |
| Fever (>38°C), n (%) | 78/112 (69.6) |
| Maximum temperature (°C), median (IQR) | 38.7 (37.8−39.3) |
| Clinical diagnosis | |
| Bronchiolitis, n (%) | 42/114 (36.8) |
| Lower respiratory tract infection, n (%) | 31/114 (27.2) |
| Bronchospasm, n (%) | 18/114 (15.8) |
| Pneumonia, n (%) | 21/114 (18.4) |
| Laryngitis, n (%) | 2/114 (1.8) |
| Personal history | |
| Congenital heart disease, n (%) | 2/114 (1.8) |
| Bronchopulmonary dysplasia, n (%) | 4/114 (3.5) |
| Preterm birth (GA <37 weeks), n (%) | 22/114 (19.3) |
| Allergic disease, n (%) | 11/92 (12.0) |
| Complex chronic condition, n (%) | 11/114 (9.7) |
| Family history and household characteristics | |
| Family history of asthma, n (%) | 24/112 (21.4) |
| Exposure to tobacco smoke, n (%) | 12/97 (12.4) |
| Siblings, n (%) | 74/113 (65.5) |
| Diagnostic tests | |
| Chest X-ray, n (%) | 57/114 (50.0) |
| Normal, n (%) | 8/57 (14.0) |
| Consolidation or effusion, n (%) | 15/57 (26.3) |
| Other type of infiltration, n (%) | 34/57 (59.6) |
| Blood culture, n (%) | 19/114 (16.7, all negative) |
| Urine culture, n (%) | 3/114 (2.6, all negative) |
| Complete blood count and chemistry, n (%) | 40/114 (35.1) |
| Hemoglobin (g/dL), median (IQR) | 11.5 (10.8−12.0) |
| Leucocytes (cells/μL), median (IQR) | 11 400 (7800−14 300) |
| Neutrophils (cells/μL), median (IQR) | 6700 (4800−8300) |
| C-reactive protein (mg/L), median (IQR) | 26.3 (12.9−76.8) |
| Procalcitonin (ng/mL), median (IQR) | 0.97 (0.30−1.66) |
| Treatment | |
| Admission to RICU, n (%) | 62/114 (54.4 %) |
| Admission to ICU, n (%) | 5/114 (4.4) |
| Oxygen therapy, n (%) | 104/114 (91.2) |
| NIV, n (%) | 47/114 (41.2) |
| Duration of NIV (days), median (IQR) | 3.0 (2.0−5.8) |
| Steroids (oral or intravenous), n (%) | 51/114 (44.7) |
| Inhaled salbutamol, n (%) | 73/114 (64.0) |
| Ipratropium, inhaled, n (%) | 32/114 (28.1) |
| Nebulized adrenaline, n (%) | 2/114 (1.8) |
| Antibiotics, n (%) | 33/114 (28.9) |
| Duration of antibiotherapy (days), median (IQR) | 4.0 (3.0−5.5) |
Abbreviations: GA, gestational age; ICU, intensive care unit; IQR, interquartile range; NIV, noninvasive ventilation; RICU, respiratory intermediate care unit; SpO2, peripheral capillary oxygen saturation.
In 54.4% of cases, the patient required admission to the respiratory intermediate care unit. Five patients were admitted to the intensive care unit, one due to respiratory failure (coinfection by Mycoplasma pneumoniae), one to febrile seizures, one to metabolic decompensation in citrullinemia, and two due to pleural effusion greater than 10mm (only one of them required surgical drainage, with negative culture results). None of the patients required invasive ventilation, and none had pulmonary bullae or abscess.
The genotype of hMPV was identified in 28 cases: type A in 17 and type B in 11, with no cases of mixed infection by types A and B. Coinfection with another microorganism was detected in 40.4%, involving rhinovirus/enterovirus (21.9%), respiratory syncytial virus (5.3%) and Mycoplasma pneumoniae (one case, 0.9%), among others.
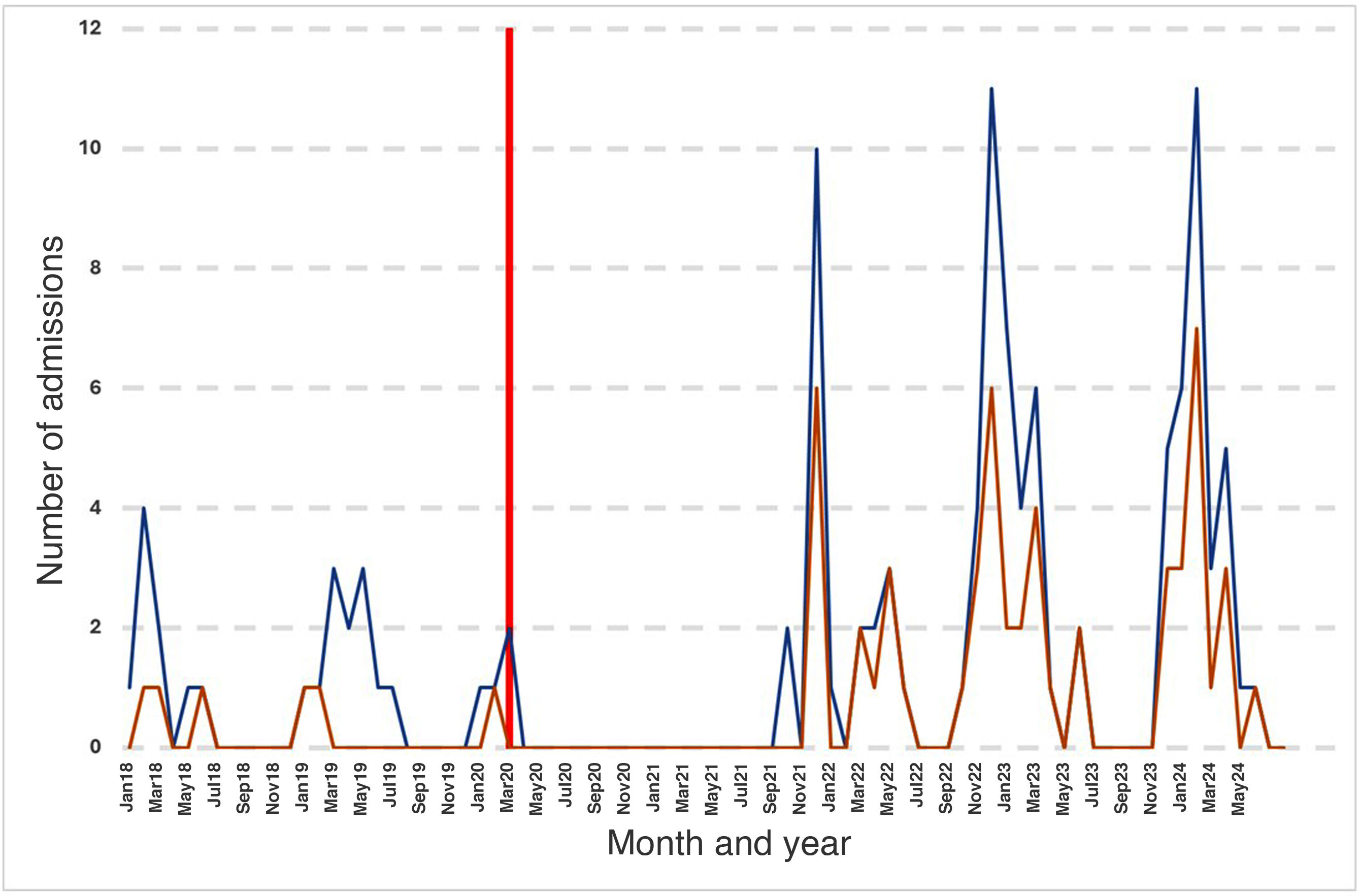
The incidence peaked in winter. December was the month with the highest number of admissions, and 71.9% of admissions occurred between December and March, with admissions practically disappearing in summer.
Following the onset of the COVID-19 pandemic, there were 18 consecutive months with no reported cases (Fig. 1). Subsequently, cases surged in the winter months, with incidences that tripled those documented before the pandemic. This trend persisted for three seasons. The increase in incidence occurred on account of infections in children aged more than 12 months, with a statistically significant difference in the median age of patients between the prepandemic and postpandemic periods (37.3 [16.2–54.8] weeks vs 67.3 [25.6–109.8] weeks; P=.015).
We identified some changes in the clinical features after the pandemic. There were no differences in the frequency of symptomatic cases or respiratory manifestations, but the maximum temperature was higher in the postpandemic compared to the prepandemic period (38.8°C [38.0–39.4] vs 38.1°C [37.0–39.0]; P=.015). There were no differences in the frequency of admission to the intensive care unit or the use of noninvasive ventilation, bronchodilators or oxygen therapy. In the postpandemic period, admission to the respiratory intermediate care unit was less frequent (49.4% vs 72.0%; P=.042) as was the use of systemic steroids (39.3% vs 64.0%; P=.028); the duration of oxygen therapy decreased (3.0 [1.8–4.3] vs 4.0 [2.8–7.0] days; P=.049), while there was increase in the use of chest X-rays (55.1% vs 32.0%; P=.040) and of antibiotics (33.7% vs 12.0%; P=.024). There was no change in the frequency of blood tests. The only difference in blood test results was an increase in neutrophil counts after the pandemic (6750 [4875−10400] vs 5400 [3500–5950] cells/μL; P=.039.
We found a difference in the clinical diagnoses: all diagnoses of pneumonia or laryngitis occurred in the postpandemic period.
Although the scope of our study is limited on account of its retrospective and single-center design, its findings confirm the seasonal pattern of hMPV infection2 and the increase in incidence in children aged more than 12 months following the COVID-19 pandemic. The latter has been attributed to an “immunity debt” resulting from the lack of transmission of communicable diseases during the pandemic.3 Disease severity did not change noticeably between pre- and postpandemic admissions. In the postpandemic period, while bronchiolitis continued to be the predominant presentation, there were cases of pneumonia and laryngitis, which had not been previously diagnosed in association with hMPV. This could be indicative of greater virulence in some circulating strains or changes in host susceptibility,4 but it is likely only a reflection of the greater age of the patients.5 In conclusion, infections by hMPV constitute a significant health care burden (admissions to respiratory intermediate care unit and intensive care unit, need of respiratory support), may present with lobar consolidation and in some cases significant pleural effusion on radiography, and the COVID-19 pandemic has been associated with a shift in incidence to older children and slight changes in the clinical presentation, without an associated increase in severity.