Investigation and control of a respiratory syncytial virus (RSV) outbreak that affected the Neonatal Intensive Care Unit (NICU) of a university hospital from October to December 2012.
Patients and methodsCohort study of children admitted to the NICU. The infection attack rate was calculated. A descriptive analysis of the cases and a multivariate analysis was performed using the variables that were shown to be risk factors for RSV infection.
Preventive measures taken were: contact isolation; hand hygiene training and observation; exclusivity of a health team of nurses and physicians for positive cases, restrictions on visitor numbers; surveillance RSV testing, and palivizumab prophylaxis.
ResultsThe outbreak had three epidemic waves and 20 positive cases out of a total of 48 children admitted. The overall attack rate was 42%. Half of positive cases were children, with a median age of 36 days (p25=22, p75=58). The independent risk factors for RSV infection were birth weight below 1000g (OR=23.5; P=.002) and to have another nosocomial infection the week before the diagnosis of RSV infection (OR=19.98; P=.016).
ConclusionsIt was an outbreak with a high number of cases, due to the delay in notification, prolonged RSV carrier status, and low adherence to hand hygiene practice, which favoured the cross-transmission of infection. The most effective preventive measures were direct observation of hand hygiene and supervision of isolation measures.
Investigación y control de un brote por virus respiratorio sincitial (VRS) que afectó a la Unidad de Neonatología (UN) de un hospital universitario de octubre a diciembre del 2012.
Pacientes y métodosEstudio de cohortes de los niños ingresados en la UN. Se calculó la tasa de ataque de infección y se realizaron un análisis descriptivo de los casos y un análisis multivariante de aquellas variables que mostraron ser factores de riesgo de infección por VRS.
Las medidas preventivas llevadas a cabo fueron: aislamiento de contacto de casos; formación y observación de higiene de manos; exclusividad del personal sanitario para casos, restricción de visitas; estudio de portadores de VRS y profilaxis con palivizumab.
ResultadosEl brote tuvo 3 ondas epidémicas y un total de 20 casos, de 48 niños ingresados. La tasa de ataque global fue del 42%. De los casos, la mitad fueron niños, con una edad mediana de 36 días (p25=22, p75=58). El peso al nacimiento inferior a 1.000g (OR=23,5; p=0,002) y tener otra infección nosocomial en la semana previa al diagnóstico de infección por VRS (OR=19,98; p=0,016), fueron factores de riesgo independientes de infección por VRS.
ConclusionesSe trató de un brote epidémico con un elevado número de casos, relacionado con el retraso en la notificación, el tiempo prolongado del estado de portador del VRS y los fallos en el cumplimiento de la higiene de manos, que favoreció la transmisión cruzada de la infección. Las medidas preventivas más eficaces fueron la observación directa de higiene de manos y supervisión de las medidas de aislamiento.
Respiratory syncytial virus (RSV) is a single-stranded RNA virus of the Paramyxoviridae family. It is classified into A and B subtypes, which may co-circulate, although subtype A generally dominates.1
Respiratory syncytial virus is the single most important cause of lower respiratory tract infections2 and the leading cause of acute bronchiolitis3 in children. It may cause upper respiratory tract infections, lower respiratory tract infections and pneumonia.4 It causes seasonal outbreaks that vary by geographical area, with its incidence peaking between November and February in Spain.5 It is one of the main causative agents in nosocomial respiratory tract infections in the paediatric population, and causes outbreaks associated with considerable morbidity and mortality, especially in patients with certain underlying diseases. Prolonged viral shedding and the potential susceptibility of patients and health care staff, since permanent immunity cannot be acquired, make it difficult to control nosocomial spread.7
The most frequent mode of transmission is direct contact, as the virus can remain for hours in surfaces and the hands of health care workers.8
In this article, we analyse an outbreak of RSV that affected 20 infants hospitalised in a neonatal unit (NU) and the measures taken to control the spread of infection.
Patients and methodsThe NU was located in a tertiary care university hospital (referral hospital).9 The paediatric department serves a catchment area with a population of 150,619 children.10
The NU, which is the referral unit for the autonomous community of Andalusia, is divided into General Neonatology (GN), which has 28 beds, 5 doctors and a nurse-patient ratio of one nurse to six or seven patients; the Intermediate Care Nursery (ICN), with 16 beds, 6 doctors and a ratio of one nurse per three or four patients; and the Neonatal Intensive Care Unit (NICU), with 12 beds, 6 doctors and a ratio of one nurse per two patients.
Definitions. We defined a suspected case of RSV infection as a neonate admitted to the NU between October 2 and December 6 that exhibited symptoms compatible with one of the following: upper respiratory tract infection (presence of cough, rhinorrhoea and/or fever); lower respiratory tract infection (hypoxaemia, stridor or wheezing on auscultation, or use of accessory muscles for breathing); pneumonia (presence of respiratory symptoms and evidence of lung consolidation in chest X-ray)4 and/or bronchiolitis (rhinitis, tachypnoea, wheezing, cough, crepitus and/or nasal flaring).3 A confirmed case was defined as a neonate admitted to the NU between October 2 and December 6 with a positive RSV antigen detection test of a nasopharyngeal wash sample and symptoms compatible with upper respiratory tract infection, lower respiratory tract infection, pneumonia and/or bronchiolitis. We defined asymptomatic carrier as a neonate admitted to the UN between October 2 and December 6, with a positive RSV antigen detection test of a nasopharyngeal aspirate sample and no apparent symptoms of respiratory infection. We calculated the length of stay in the NU for RSV cases as the number of days elapsed from admission to the day of microbiological diagnosis. For those that did not become ill, length of stay was calculated as the days elapsed from admission to the day they underwent screening. We used the World Health Organisation (WHO) classification11 of preterm birth and its subcategories based on gestational age (GA), thus defining preterm birth as a birth before 37 weeks’ gestation, moderate to late preterm as a birth between 32 and 37 weeks’ gestation, very preterm as a birth between 28 and 32 weeks’ gestation, and extremely preterm as a birth before 28 weeks’ gestation.
Microbiological testing. The diagnosis of RSV was made based on the detection of the viral antigen in nasopharyngeal lavage samples by chromatographic immunoassay (BD Directigen EZ RSV®; New Jersey, USA). This test has a reproducibility of 99.1%, a sensitivity of 80% and a specificity of 91%.
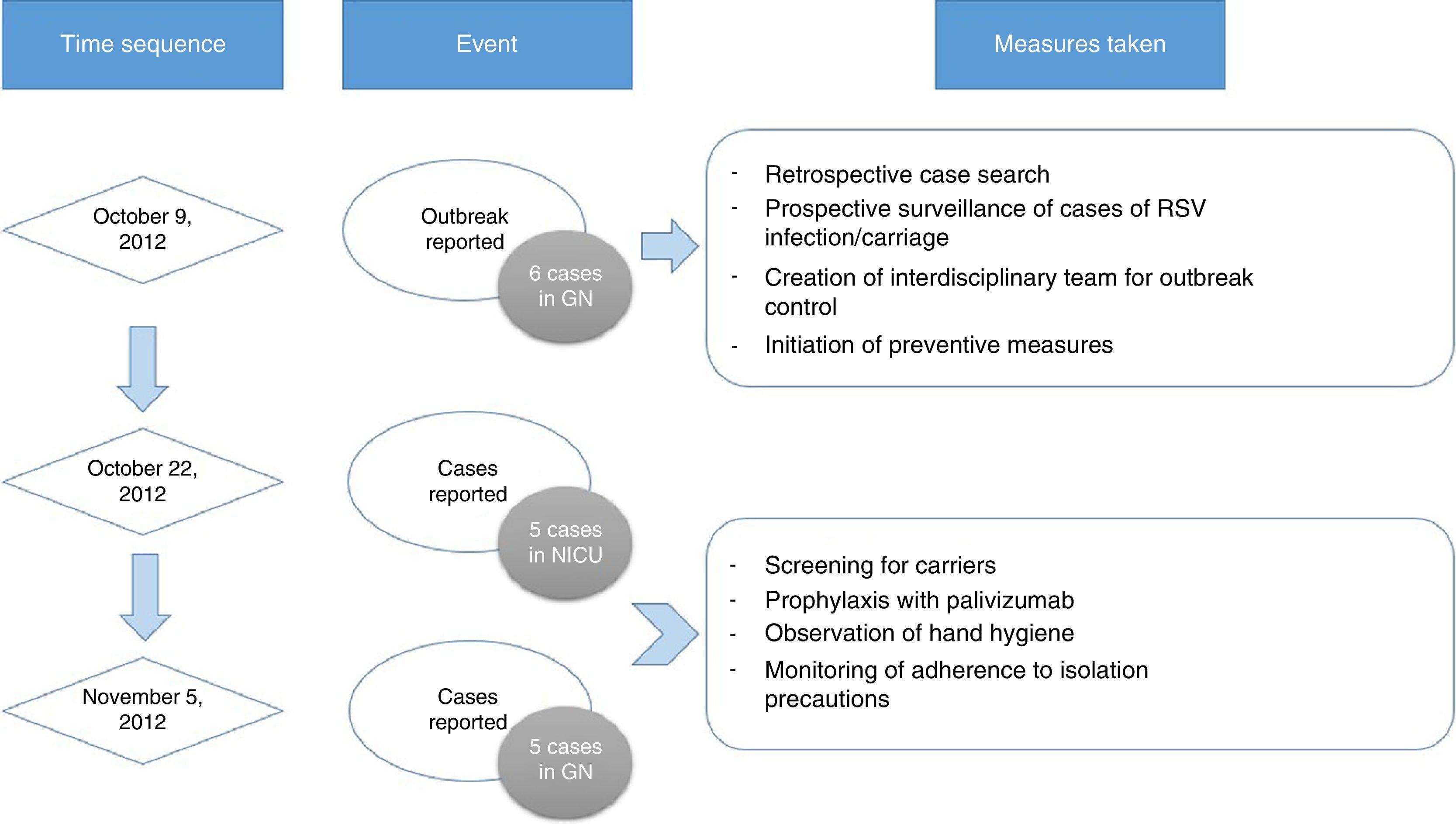
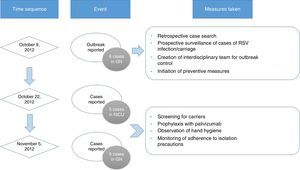
Outbreak detection and measures for intervention. On October 9, 2012, the chief of the NU notified the department of preventive medicine of the presence of six cases of RSV infection in GN. Subsequently, a retrospective search and prospective surveillance for additional cases was initiated, an interdisciplinary team was created to control the outbreak, and the following preventive measures were implemented: contact isolation of patients suspected to be infected by RSV based on their clinical presentation, with particular emphasis on handwashing and strict compliance with the hand hygiene recommendations of the WHO,12 care of infected patients by exclusively-dedicated health care staff, and visitation restrictions.
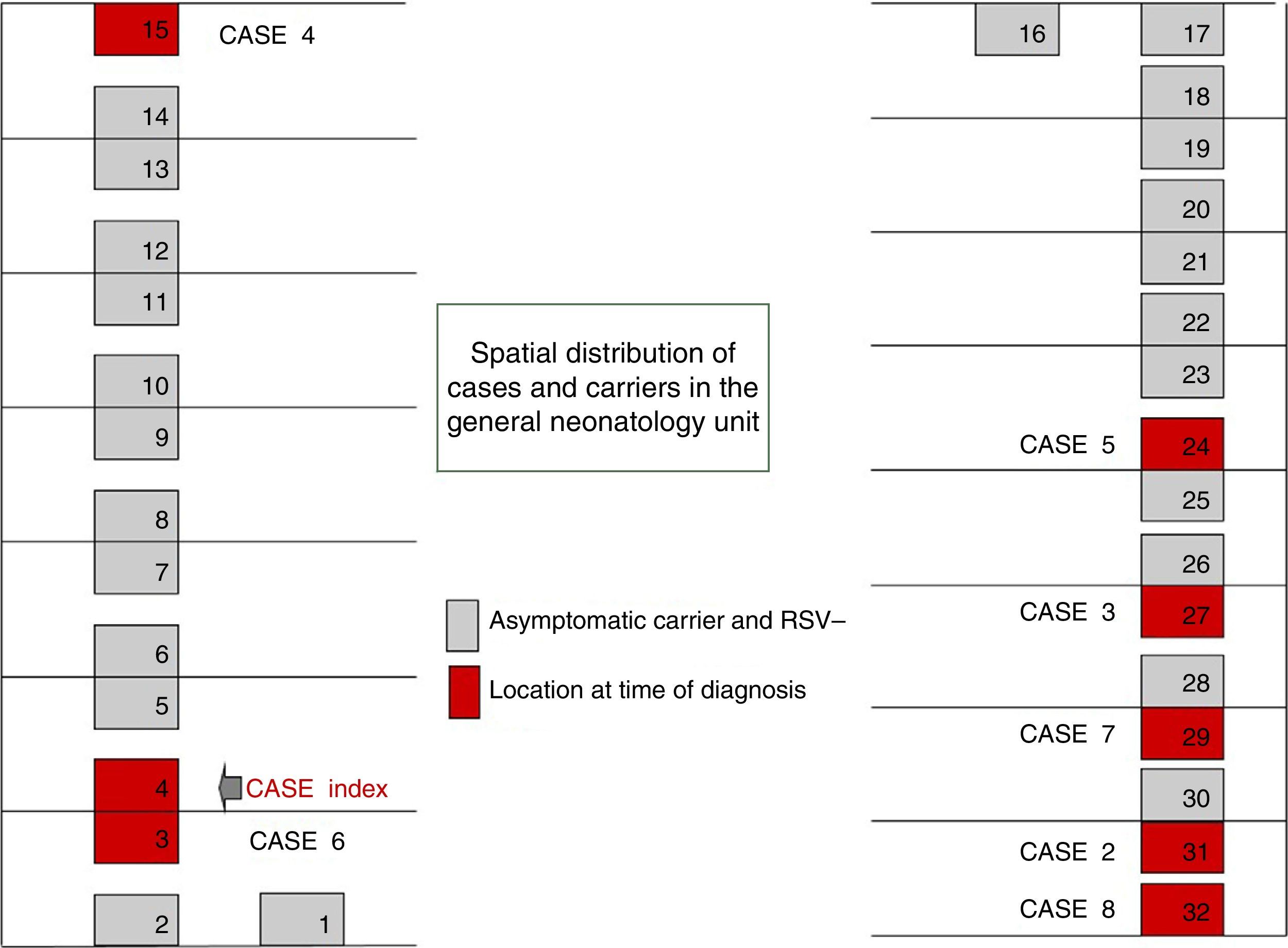
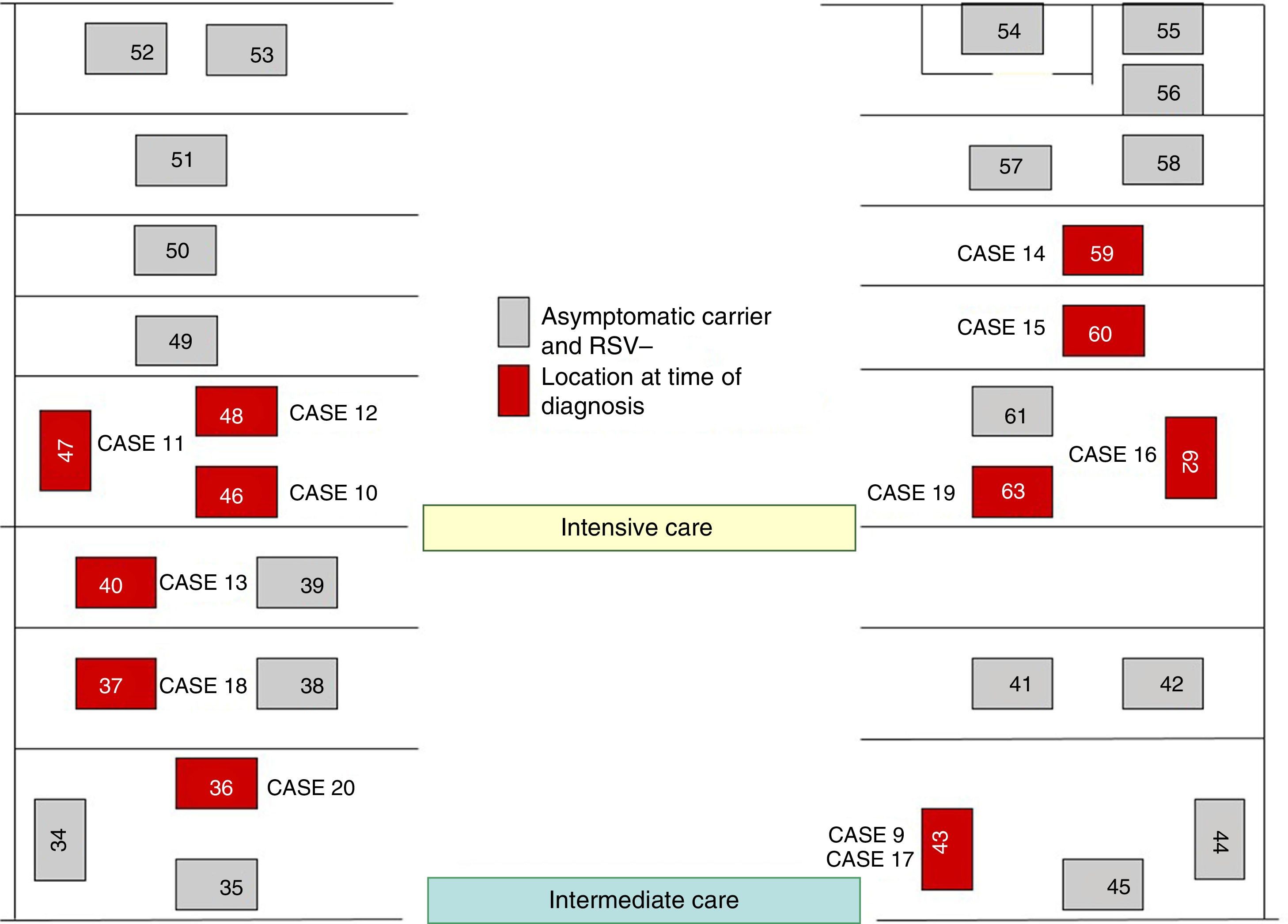
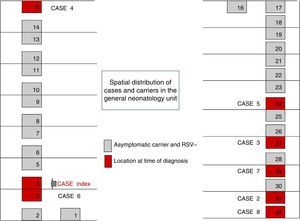
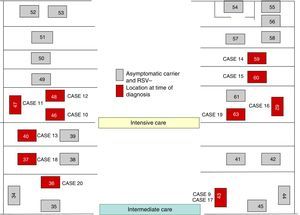
On October 22 and November 5, new cases were detected in the NICU and in GN, respectively, and further measures were implemented, including RSV screening for identification of carriers, prophylaxis with palivizumab, and monitoring of hand hygiene and compliance with isolation measures in all three units. Figs. 1 and 2 show the distribution of disease cases and infected patients in the affected units, while Fig. 3 summarises the flow of events.
Study design. We studied the cohorts consisting of all the neonates hospitalised in the NU from the time the first case was diagnosed (October 2, 2012) to the time the last two cases were detected (November 6, 2012).
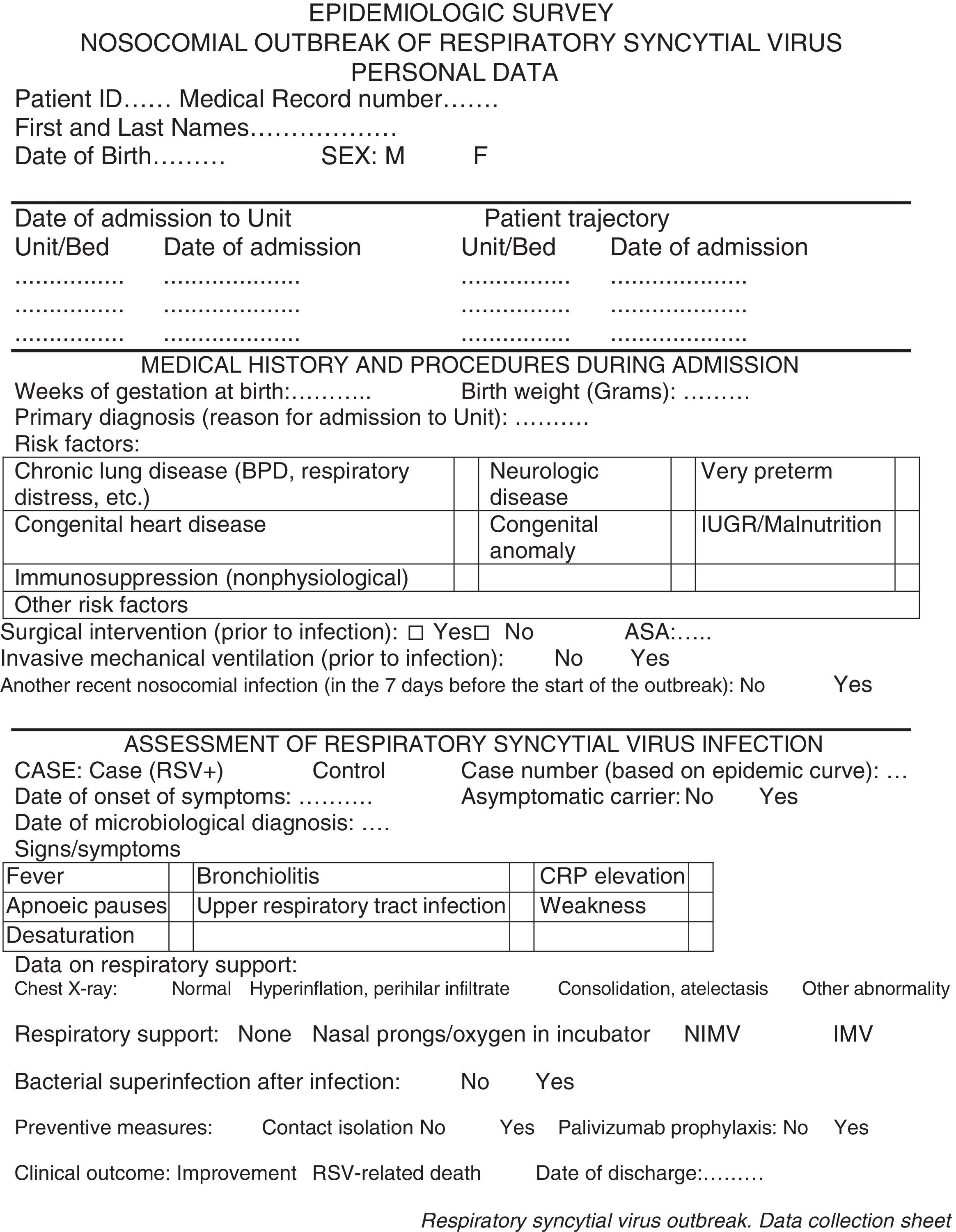
Data collection. We used the protocolized individual questionnaire developed by the Department of Preventive Medicine (see Appendix A) to collect personal information and data for variables concerning the disease process, medical history, and interventions performed.
Statistical analysis. We calculated the overall RSV attack rate and the attack rate for each unit, which corresponds to the probability that patients hospitalised in the unit get infected by RSV. We defined at-risk individual as a neonate hospitalised in the NU during the outbreak.
We performed a univariate descriptive statistical analysis, summarising categorical variables as frequency distributions and quantitative variables as measures of central tendency and dispersion. For the bivariate analysis, we assessed the associations between variables using the chi square test or Fisher's exact test. Statistical significance was defined as P<.05. We used the Mann–Whitney U test for independent samples to compare the medians of two groups. We used logistic regression for the multivariate analysis of variables previously identified as risk factors for RSV infection. We analysed the data using the Statistical Package for the Social Sciences (SPSS) version 20 (IBM; Armonk, New York, USA).
The variables described in the univariate and bivariate analyses were: age at diagnosis of RSV infection; length of stay in the unit until diagnosis of RSV infection; birth weight (in grams); GA (in weeks); degree of prematurity; and positive history of chronic lung disease, heart disease, patent ductus arteriosus, neurologic disease, congenital anomaly, intrauterine growth restriction (IUGR) and malnutrition, surgery prior to diagnosis of RSV infection, mechanical ventilation prior to diagnosis of RSV infection, and nosocomial infection in the seven days preceding the diagnosis of RSV infection.
The variables corresponding to P values of less than 0.05 in the bivariate analysis that were included in the multivariate analysis were GA, birth weight, length of stay in the unit, history of chronic lung disease, prior nosocomial infection and degree of prematurity.
ResultsOutbreak characteristicsThe overall RSV attack rate was 42%, with attack rates of 38% for GN, 42% for the ICN and 47% for the NICU (Table 1).
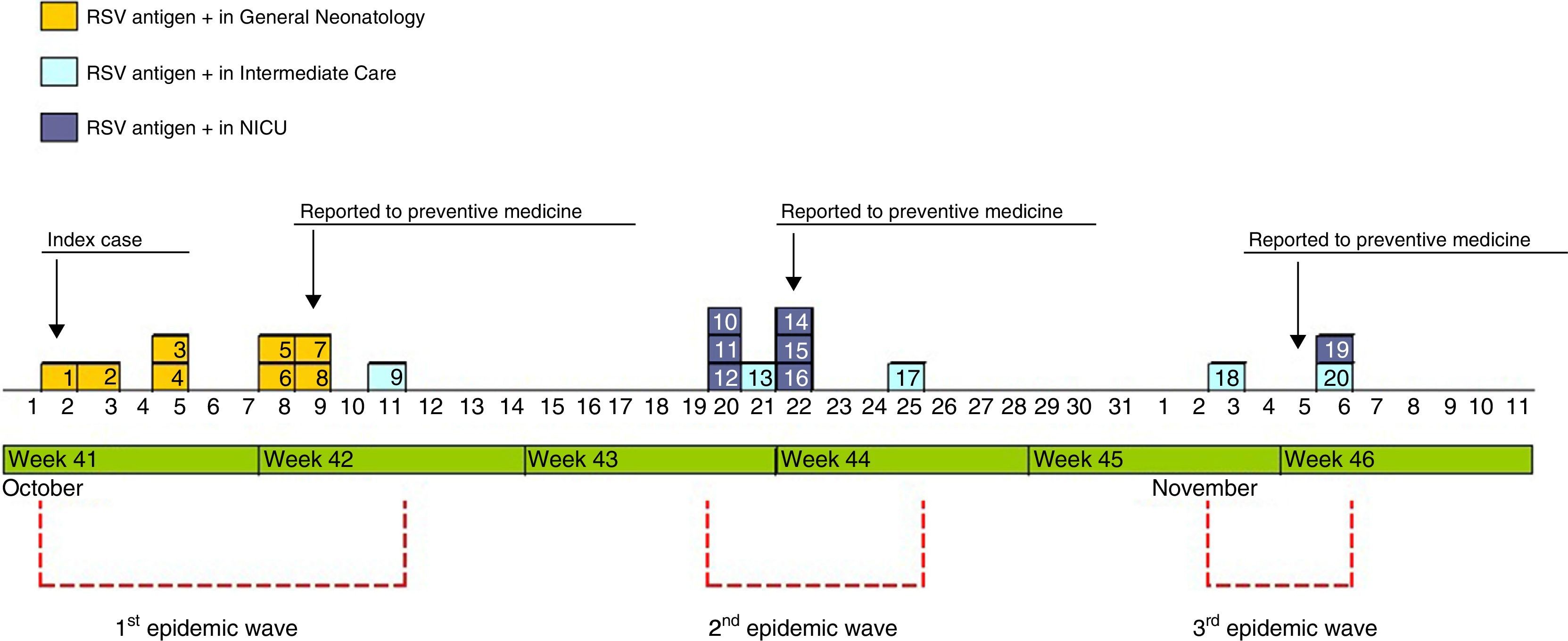
The outbreak comprised three epidemic waves and a total of 20 cases in the 48 hospitalised neonates (Fig. 4). The first wave started on October 2 in GN and comprised 9 cases. The first case occurred in a patient admitted to the ICU on August 6 and transferred to GN on September 28, in whom testing of a nasopharyngeal aspirate sample detected the presence of RSV antigen on October 2. The second wave affected the NICU and ICN, occurred between October 20 and 25, and comprised eight cases. The third wave, from December 3 to 6, comprised the three remaining cases detected in the NICU and ICN.
Of the total number of cases, twelve were reported by GN in the course of the three epidemic waves, and the other eight cases were detected in the screening for carriers.
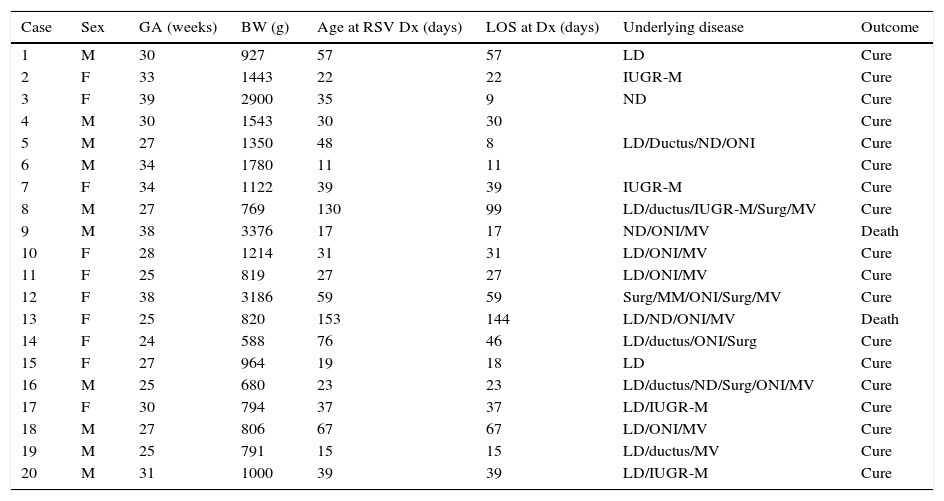
Of the 20 cases, half corresponded to male and half to female patients, with a median age at diagnosis of RSV infection of 36 days (IQR, 22–58), a median gestational age of 29 weeks (IQR, 25.5–33.8) and a median birth weight of 982g (IQR, 797–1518). The mean length of stay in the unit was 30.5 days (IQR, 17.25–54.25) (Table 2). As for the clinical manifestations, out of the 20 patients, 15 developed symptoms (75%), albeit mild ones, and only 30% of symptomatic patients developed a full case of bronchiolitis. Radiologic findings were normal in most patients (60%); there were signs of hyperinflation or perihilar infiltrates in 25%, and signs of lung consolidation or atelectasis in 15%.
Characteristics of patients with tests positive for respiratory syncytial virus infection.
| Case | Sex | GA (weeks) | BW (g) | Age at RSV Dx (days) | LOS at Dx (days) | Underlying disease | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | M | 30 | 927 | 57 | 57 | LD | Cure |
| 2 | F | 33 | 1443 | 22 | 22 | IUGR-M | Cure |
| 3 | F | 39 | 2900 | 35 | 9 | ND | Cure |
| 4 | M | 30 | 1543 | 30 | 30 | Cure | |
| 5 | M | 27 | 1350 | 48 | 8 | LD/Ductus/ND/ONI | Cure |
| 6 | M | 34 | 1780 | 11 | 11 | Cure | |
| 7 | F | 34 | 1122 | 39 | 39 | IUGR-M | Cure |
| 8 | M | 27 | 769 | 130 | 99 | LD/ductus/IUGR-M/Surg/MV | Cure |
| 9 | M | 38 | 3376 | 17 | 17 | ND/ONI/MV | Death |
| 10 | F | 28 | 1214 | 31 | 31 | LD/ONI/MV | Cure |
| 11 | F | 25 | 819 | 27 | 27 | LD/ONI/MV | Cure |
| 12 | F | 38 | 3186 | 59 | 59 | Surg/MM/ONI/Surg/MV | Cure |
| 13 | F | 25 | 820 | 153 | 144 | LD/ND/ONI/MV | Death |
| 14 | F | 24 | 588 | 76 | 46 | LD/ductus/ONI/Surg | Cure |
| 15 | F | 27 | 964 | 19 | 18 | LD | Cure |
| 16 | M | 25 | 680 | 23 | 23 | LD/ductus/ND/Surg/ONI/MV | Cure |
| 17 | F | 30 | 794 | 37 | 37 | LD/IUGR-M | Cure |
| 18 | M | 27 | 806 | 67 | 67 | LD/ONI/MV | Cure |
| 19 | M | 25 | 791 | 15 | 15 | LD/ductus/MV | Cure |
| 20 | M | 31 | 1000 | 39 | 39 | LD/IUGR-M | Cure |
BW, birth weight; Dx, diagnosis; F, female; GA, gestational age; HD, heart disease; IUGR-M, intrauterine growth restriction-malnutrition; LD, lung disease; LOS, length of stay in the unit; M, male; MM, multiple malformation; MV, invasive mechanical ventilation prior to RSV infection; ND, neurologic disease; ONI, other nosocomial infection; RSV, respiratory syncytial virus; Surg, surgery prior to RSV infection.
The hospitalised neonates that were not infected by RSV were aged a median of 13.50 days (IQR, 5.5–24.5), with a median GA of 36 weeks (IQR, 32–38), a median birth weight of 2785g (IQR, 1966–3565) and a length of stay in the unit of 8.5 days (IQR, 4.25–21). The bivariate analysis showed that the differences in birth weight, GA and length of stay between cases and patients that did not get infected were statistically significant for all three variables (P<.001).
Eighty-five percent of cases were born preterm, compared to fifty-seven percent of controls. Twenty-five percent of cases were very preterm and forty-five percent extremely preterm, and we found a statistically significant association between RSV infection and very preterm birth (P=.029), and between RSV infection and extremely preterm birth (P=.013). Sixty-five percent of cases had a history of chronic lung disease compared to twenty-nine percent of controls (P=.012). Forty percent of cases had another nosocomial infection in the week preceding the diagnosis of RSV infection compared to 3.6% of controls (P=.002).
Fifty percent of cases received prophylactic treatment with monoclonal antibodies (palivizumab) compared to twenty-nine percent of controls.
As for the clinical outcomes, most patients were discharged after improving, two of the cases (10%) and one of the patients that was not infected by RSV (3.6%) died. The cause of death was unrelated to RSV infection in all three.
We did not find a statistically significant association between RSV infection and a positive history of heart disease, patent ductus arteriosus, neurologic disease, congenital anomaly, IUGR, surgery prior to diagnosis of RSV infection or mechanical ventilation prior to diagnosis of RSV infection.
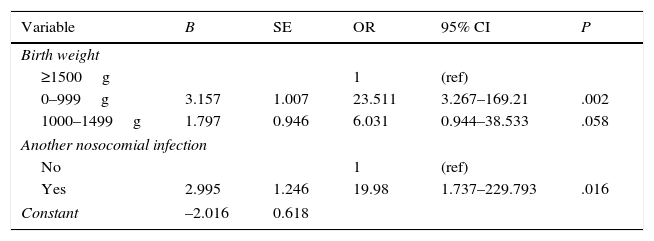
The multivariate logistic regression analysis (Table 3) identified the following as independent risk factors for infection by RSV: birth weight of less than 1000g 1 (OR=23.5; 95% CI, 3.26–169.21; P=.002) and having had another nosocomial infection in the week preceding the diagnosis of RSV infection (OR=19.98; 95% CI, 1.73–229.79; P=.016). The resulting model classified 81.3% of the analysed cases correctly, and the variability in infection by RSV virus (independent variable) predicted by the model ranged from 39.7% (Cox and Snell R square) to 53.5% (Nagerkelke's R square).
Multivariate logistic regression. Independent risk factors for infection by respiratory syncytial virus.
| Variable | B | SE | OR | 95% CI | P |
|---|---|---|---|---|---|
| Birth weight | |||||
| ≥1500g | 1 | (ref) | |||
| 0–999g | 3.157 | 1.007 | 23.511 | 3.267–169.21 | .002 |
| 1000–1499g | 1.797 | 0.946 | 6.031 | 0.944–38.533 | .058 |
| Another nosocomial infection | |||||
| No | 1 | (ref) | |||
| Yes | 2.995 | 1.246 | 19.98 | 1.737–229.793 | .016 |
| Constant | –2.016 | 0.618 | |||
Respiratory syncytial virus is the leading cause of lower respiratory tract infection in infants younger than one year,13,14 it is one of the most frequent causative pathogens in outbreaks of nosocomial infection, and is associated with a high morbidity and mortality.15,16
In infants aged less than 6 months, chronic lung disease, birth before 35 weeks’ gestation, congenital heart disease and immunodeficiency are well-known risk factors for RSV infection.17,18 Preterm newborns are most susceptible to infection by RSV due to the immaturity of their immune system and their low serum levels of maternal antibodies.15,19 In our study, the average GA and birth weight of neonates infected by RSV were significantly lower than those of the neonates that were not infected, which was consistent with the results of previous studies.8,20 We also found a significant association between RSV infection and chronic lung disease, which has also been described in the literature.15,17 Infection by RSV was associated with a longer length of stay in the unit until diagnosis or screening, but we did not find an association with other risk factors reported in previous studies, such as underlying congenital heart disease.21 We also found a significant association between RSV infection and having had another nosocomial infection in the week prior to its diagnosis. This could be explained by a weakened immune system in these children, which would make them more susceptible to infection by another pathogen, although we did not find data to support this hypothesis in the literature. The multivariate logistic regression analysis only identified two independent risk factors for becoming infected by RSV, which were birth weight of less than 1000g and having had another nosocomial infection in the week preceding the diagnosis of RSV infection. The degree of prematurity variable was a potential confounding factor due to its close association with birth weight, and was therefore removed from the logistic regression model.
The mortality of patients with community-acquired RSV infection is negligible, unlike that of patients with nosocomial infections, which can be considerable.22 The risk of death is higher in children of younger ages, born preterm, or with congenital heart disease, bronchopulmonary dysplasia or immunodeficiency, as they are more susceptible to severe disease.6 In our study, the three deaths that occurred in the patients under study were unrelated to RSV infection.
Thirty percent of the cases required invasive mechanical ventilation, but since all these patients had required it prior to the diagnosis of RSV infection, we were unable to determine its association with RSV.
Early detection of cases is a crucial measure in outbreak control, as it allows the timely implementation of appropriate isolation precautions and the containment of viral transmission. Respiratory syncytial virus spreads through direct contact or indirectly through fomites, and it can survive on inorganic surfaces for up to 12h, which prolongs the time span during which transmission is possible.8,21 In addition, while the usual duration of viral shedding is two weeks, this period can be longer in premature or immunocompromised infants, and therefore all patients should be tested when an outbreak of RSV is suspected to detect potential asymptomatic cases of infection. For all the above reasons, preventive measures such as screening for virus carriage are not only important for the detection of new cases, but can be helpful in estimating how long contact isolation precautions should be maintained.8
One of the limitations of our study was that the respiratory secretion samples of the patients were not analysed with polymerase chain reaction techniques. This may have resulted in underdiagnosis of RSV infection, as the sensitivity of the chromatographic immune assays that were used is lower. Furthermore, sequencing the gene products obtained by amplification would allow additional serogroup and molecular epidemiology analyses, which could not be conducted in this study. However, the availability of PCR would not have changed the preventive measures used to control the outbreak in any significant way.
It is worth highlighting the considerable number of cases detected in our study. Some of the factors that may have contributed to it are the screening for carriers (which identified 8 of the 20 diagnosed cases), the delay in reporting (there were already six cases of RSV infection at the time of the first report), the early discontinuation of isolation precautions (which were automatically discontinued seven days after their initiation), and gaps in the adherence to hand hygiene measures.
Despite the delay in reporting, which was due to flaws in the internal communication between the involved departments, the creation of the interdisciplinary team helped address these issues and facilitated the quick implementation of preventive measures, which was crucial in the management and containment of the outbreak. These precautions, such as the isolation of infected patients, the screening for carrier detection, the assignment of an exclusively-dedicated staff to the care of patients infected by RSV, and the emphasis placed on the adherence to appropriate hand hygiene, were measures that are generally recommended for infection control.6,21,23–25 Furthermore, following the recommendations given in different studies26,27 and clinical practice guidelines,28,29 adherence to hand hygiene was assessed by direct observation of the performance of health care workers during their shifts and the supervision of isolation precautions. In our study, the adherence of health care workers to hand hygiene measures was of 63%, a percentage that exceeded the 40% reported in a recent systematic review.30 However, we must take into account that the monitoring took place during the study of the outbreak, which may have increased the motivation of health care workers to strictly adhere to hand hygiene measures. Of all the measures that were implemented, those that seemed to be most effective were the observation of hand hygiene and the supervision of isolation precautions, as no further cases were detected after they started. In contrast, the screening for carriers did succeed in detecting new cases but did not lead to the end of the outbreak.
In conclusion, this was an outbreak with a large number of cases, a fact that was associated with delayed reporting and the long duration of RSV carriage, and further facilitated by flaws in the hand hygiene of the health care staff and in contact isolation precautions.
The experience gained during this outbreak allows us to conclude that the creation of interdisciplinary teams is key in the control of epidemic outbreaks, as it leads to the quick and consistent implementation of preventive measures. Furthermore, we believe that such teams should be permanent in high-risk units, as they provide effective channels of communication between the departments involved in the daily surveillance of health care-related infections.
Conflict of interestsThe authors have no conflict of interests to declare
Please cite this article as: Parejo CM, García AM, Domínguez CL, Ochoa CC, Martín JA, Herrera MC. Brote por virus respiratorio sincitial en la Unidad de Neonatología de un hospital de tercer nivel. An Pediatr (Barc). 2016;85:119–127.
Previous presentations: This study was presented as an oral communication (Brote nosocomial de infección por virus respiratorio sincitial en una Unidad de Neonatología) at the Congreso Iberoamericano de Epidemiología y Salud Pública, XXXI Reunión Científica de la Sociedad Española de Epidemiología; September 4–6, 2013; Granada, Spain.