Acquired bone marrow aplasia is a disorder with an estimated incidence of 1–2 cases per million inhabitants per year1 characterised by low bone marrow cellularity that results in pancytopaenia. The first-line treatment in children is matched related donor haematopoietic stem cell transplantation (HSCT), and if this option is not available, immunosuppressive therapy2; should the latter fail, the next step is matched unrelated donor HSCT. Eltrombopag, a thrombopoietin analogue, has been recently introduced as a possible treatment of refractory aplasia. There is published evidence of its effectiveness in adults, but its role in paediatric patients has yet to be evaluated.
We present the case of a girl aged 11 years with severe acquired bone marrow aplasia whose test results showed trilineage peripheral blood recovery after starting treatment with eltrombopag.
The patient initially presented with generalised ecchymosis and petechiae, asthenia and metrorrhagia of 15 days’ duration. The blood panel revealed severe pancytopaenia with findings suggestive of hyporegenerative anaemia (haemoglobin, 7.6g/dL; reticulocytes, 0.69%), a platelet count of 8×1000/μL and a neutrophil count of 0.5×1000/μL, with no abnormalities in blood chemistry or findings suggestive of haemolysis or coagulopathy. There was no excess of blasts in the peripheral blood smear and serology tests were negative. The patient underwent bone marrow aspiration and biopsy, the findings of which were compatible with severe bone marrow aplasia. We ruled out paroxysmal nocturnal haemoglobinuria, and the chromosome fragility test was negative.
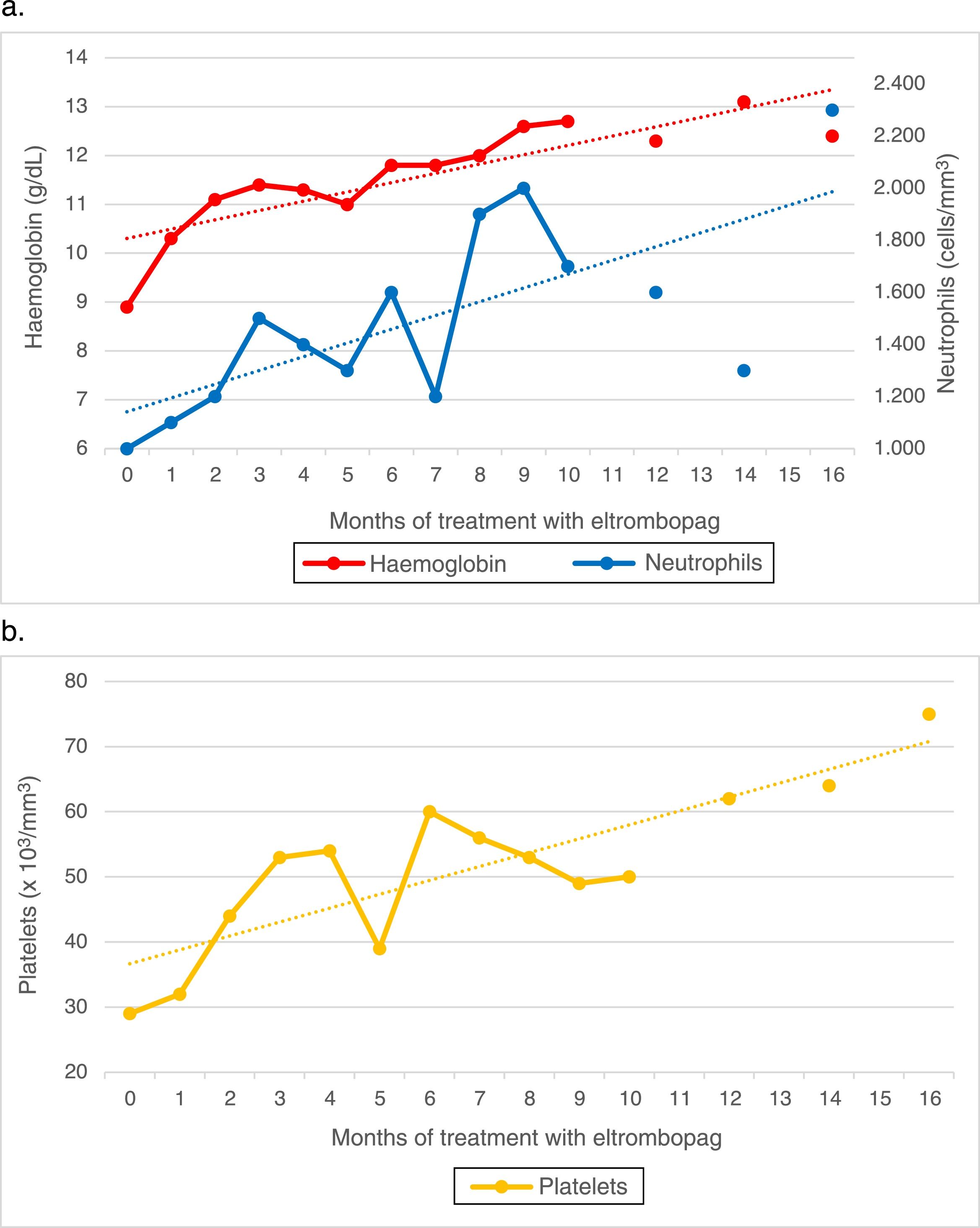
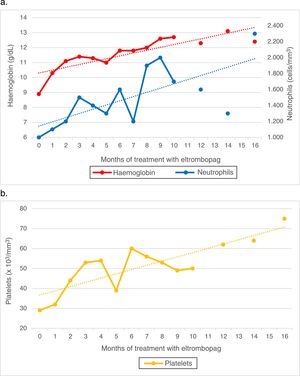
Since the patient did not have an HLA-matched relative, she started immunosuppressive therapy with thymoglobulin and ciclosporin combined with granulocyte-colony stimulating factor (G-CSF). The initial response was good, and the patient showed a favourable partial response at day 60 of treatment. She later exhibited progressive deterioration, and by day 120 the findings of the evaluation were consistent with a nonresponder profile, with the patient requiring weekly transfusions. Due to the failure of immunosuppressive therapy, the patient was considered eligible for unrelated-donor HSCT given the lack of a related donor. After an unsuccessful search for a potential donor that lasted 6 months, given the severity of her bone marrow aplasia, the decision was made to initiate treatment with eltrombopag with a dose of 50mg administered orally every 24h. The laboratory tests at 3 weeks already evinced a trilineage response, and from week 7, sustained levels of haemoglobin of 12g/dL and sustained counts of at least 50000platelets/μL and at least 1500neutrophils/μL. On week 15 we started tapering off the dose of ciclosporin, which was eventually discontinued on week 44 with no evidence of an impact on cell counts.
At present, after 16 months of treatment with eltrombopag at a dose of 50mg/24h, 5 of them as monotherapy, the follow-up bone marrow workup shows mildly low counts with increased counts of all haematopoietic stem cell lineages compared to previous evaluations. Peripheral blood values remained stable (Fig. 1). The patient has not exhibited any adverse effects at any point during the follow-up.
Acquired idiopathic bone marrow aplasia is the most frequent form of aplasia in children. In severe cases, like the one presented here, it can be life-threatening due to the risk of haemorrhage and infection, and it is essential that optimal treatment is initiated early. In our patient, management started with the first-line treatment, consisting of a combination of thymoglobulin and ciclosporin,3 but she turned out to be part of the 30% of patients that do not respond to this therapy. Several approaches have been tried in this group of patients. The first clinical trial of eltrombopag for treatment of bone marrow aplasia was conducted by Olnes et al. in 2012 in adult patients. Subsequent trials have corroborated the beneficial role of this thrombopoietin analogue in increasing the counts of not only platelets, but also the three lineages of peripheral blood cells4 in adult patients with bone marrow aplasia. In the paediatric population, clinical trials of eltrombopag have only been performed in patients with chronic primary immune thrombocytopaenia5 or with failure of platelet recovery following HSCT,6 with favourable outcomes in both. The current literature does not offer any data of its use in children with idiopathic bone marrow aplasia.
The case presented here is relevant because it is the first to illustrate the effectiveness of this drug in the treatment of paediatric bone marrow aplasia, with a sustained cellular response that persisted after one year of treatment. We ought to highlight that we found no evidence of adverse effects in the 16-month follow-up, although this time frame was too short to assess long-term toxicity.
Although our findings suggest that eltrombopag or other thrombopoietin receptor agonists may provide an alternative approach to the treatment of this disease in children, specific clinical trials need to be conducted to confirm this hypothesis.
Please cite this article as: Rubio-San-Simón A, Rodríguez IL, Gómez FV, Vivanco Martínez JL, Alonso VP. Eficacia y seguridad en el uso del eltrombopag en un caso de aplasia medular adquirida grave. An Pediatr (Barc). 2019;90:246–247.