The purpose of this study was to assess the neonatal morbidity and mortality associated with vacuum-assisted vaginal deliveries compared to all other vaginal deliveries, and to identify the associated risk factors.
Material and methodsWe conducted a retrospective case–control study in a terciary maternity hospital between 2012 and 2016, including 1802 vacuum-assisted vaginal deliveries and 2 control groups: 1802 spontaneous deliveries and 909 forceps-assisted deliveries. We considered minor complications (soft tissue trauma, cephalohaematoma, jaundice, intensive phototherapy, transient brachial plexus injury) and major complications (hypoxic-ischaemic encephalopathy, intracranial and subgaleal haemorrhage, seizures, cranial fracture, permanent brachial plexus injury), admission to the neonatal intensive care unit and death.
ResultsThe risk of soft tissue trauma (aOR, 2.4; P<.001), cephalohaematoma (aOR, 5.5; P<.001), jaundice (aOR, 4.4; P<.001), intensive phototherapy (aOR, 2.1; P<.001) and transient brachial plexus injury (aOR; 2.1, P=.006) was higher in vacuum deliveries compared to spontaneous deliveries. Admission to the neonatal intensive care unit was also higher in vacuum deliveries compared to spontaneous deliveries (OR, 1.9; P=.001). When we compared vacuum with forceps deliveries, we found a higher risk of soft tissue trauma (OR, 2.1; P=.004), cephalohaematoma (OR, 2.2, P=.046) and jaundice (OR, 1.4; P=.012). Major complications were more frequent in the vacuum group comparing with the control groups, but the difference was not significant. The 2 deaths occurred in vacuum deliveries (1.1 per 1000).
ConclusionThe proportion of minor neonatal complications was higher in the vacuum-assisted delivery group. Although major complications and death were also more frequent, they were uncommon, with no significant differences compared to the other groups. There are obstetrical indications for vacuum delivery, but these alert to the need to watch for potential neonatal complications.
El objetivo fue comparar la morbimortalidad neonatal de los partos vaginales por ventosa con los restantes partos vaginales e identificar los factores de riesgo.
Material y métodosRealizamos un estudio caso-control, retrospectivo, en un hospital materno terciario con servicios de neonatología y cuidados intensivos neonatales, entre 2012 y 2016, con inclusión de 1.802 partos vaginales con ventosa, 1.802 partos eutócicos y 909 partos con fórceps. Se consideraron complicaciones menores (traumatismo de tejidos blandos, cefalohematoma, ictericia, fototerapia doble, lesión transitoria del plexo braquial) y mayores (encefalopatía hipóxico-isquémica, hemorragia intracraneal y subgaleal, convulsión, fractura craneal, lesión permanente del plexo braquial), ingreso en la Unidad de Cuidados Intensivos Neonatales y fallecimiento.
ResultadosEl riesgo de traumatismo de los tejidos blandos (ORa 2,4; p<0,001), cefalohematoma (ORa 5,5; p<0,001), ictericia (ORa 4,4; p<0,001), fototerapia doble (ORa 2,1; p<0,001) y lesión transitoria del plexo braquial (ORa 2,1; p=0,006) fue mayor en los partos con ventosa en comparación con los eutócicos. El ingreso en la Unidad de Cuidados Intensivos Neonatales también fue mayor en los partos con ventosa que en los eutócicos (OR 1,9; p=0,001). En comparación con los partos con fórceps, también se ha comprobado un mayor riesgo de traumatismo de los tejidos blandos (OR 2,1; p=0,004), cefalohematoma (OR 2,2; p=0,046) e ictericia (OR 1,4; p=0,012) en los partos con ventosa. Los partos con ventosa presentaron mayor incidencia de complicaciones mayores que los restantes partos vaginales, pero la diferencia no fue significativa. Las 2muertes ocurrieron en partos con ventosa (1,1 por 1.000).
ConclusiónLas tasas de complicaciones neonatales menores fueron más altas en el parto con ventosa. Aunque las complicaciones mayores y la muerte también fueron más frecuentes, fueron poco comunes y no mostraron diferencias significativas. El parto con ventosa es una técnica con indicación obstétrica, pero que debe alertar sobre la necesidad de vigilancia de posibles complicaciones neonatales.
The frequency of assisted vaginal deliveries depends on current obstetric practices and varies over time and between countries.1 Some studies report decreasing rates of assisted vaginal deliveries.2 However, vacuum-assisted vaginal delivery is still a common obstetric procedure3 and seems to have replaced forceps-assisted delivery.4–6 The preference for vacuum-assisted deliveries over forceps-assisted deliveries is due to the reduced incidence of maternal trauma associated to vacuum delivery,7–10 but many studies have linked this technique to a higher incidence of neonatal short-term complications.5,7–9,11–14 Some studies have found a correlation between poor neonatal outcomes in vacuum-assisted deliveries and poor technique15 or other risk factors such as dislodgment of the cup or nulliparity.16,17 There is also literature showing no differences in the incidence of neonatal complications between vacuum-assisted deliveries and forceps-assisted deliveries18 or between assisted vaginal delivery and caesarean delivery.1 The methodology used in the studies is not comparable, with some studies including preterm deliveries.
The most common neonatal complications associated with vacuum-assisted deliveries, such as caput succedaneum, cephalohaematoma, scalp oedema and abrasions and retinal haemorrhage are not considered of major clinical relevance as they resolve spontaneously without treatment. The absolute risk of severe neurologic morbidity, such intracranial haemorrhage or brachial plexus injury, is low,16 but some like subgaleal haematoma may threaten the life of the newborn and require early identification and prompt management by the neonatologist. The evidence linking these outcomes with vacuum-assisted delivery is less clear, with studies reporting variable rates of cerebral haemorrhage and neonatal seizures.5,15,16,19
Since neonatal morbidity has been described in association with vacuum delivery, clinical observation of neonates is a common practice after delivery by this method. Neonatologists are concerned about some of these complications, particularly intracranial injuries, but the evidence on the subject is inconsistent.
We aimed to compare the neonatal morbidity and mortality associated with vacuum-assisted vaginal delivery compared to forceps-assisted vaginal delivery and spontaneous vaginal delivery (with and without obstetric intervention) in term singleton neonates.
Materials and methodsWe conducted a retrospective case–control study by reviewing the health records of all attempted vaginal deliveries at a hospital in the centre of Portugal offering specialised and intensive neonatal care services, between January 2012 and December 2016. The data were collected yearly by a team of experienced professionals in our hospital.
The main outcome of the study was the morbidity and mortality associated with vacuum-assisted vaginal deliveries, and we compared the incidence of minor and major complications associated with vacuum-assisted delivery compared to other vaginal deliveries (spontaneous and forceps-assisted).
All maternity care providers at the hospital, including residents and nurses, are trained to manage spontaneous deliveries. Assisted vaginal deliveries are only performed by obstetricians and medical residents under supervision. The decision to perform an assisted vaginal delivery and the choice of instrument is made by a consulting obstetrician and may be considered only at full dilatation. The main indications for assisted vaginal delivery were absence of progression of the second stage of labour, foetal distress or maternal indications such as exhaustion. None of the assisted deliveries were elective procedures. The system used for vacuum delivery in our hospital is the Kiwi Omnicup.
The inclusion criteria were term delivery (37–42 weeks), singleton pregnancy, cephalic presentation, and second stage of vaginal delivery. The exclusion criteria were foetal or neonatal anomalies (such as foetal growth problems, congenital heart diseases, surgical diseases) and cases involving two modes of assisted delivery (i.e., double-instrumental delivery or failed assisted delivery followed by caesarean section). We did not include cases in which data for one or more variables were not available in the records.
For controls, we selected the forceps-assisted delivery and the spontaneous vaginal delivery managed by the same team immediately preceding each vacuum delivery.
We analysed the following maternal characteristics: age, parity and maternal disease (maternal hypertension, gestational diabetes and preeclampsia). The neonatal characteristics under study were gestational age, sex and birth weight. We considered the following minor neonatal complications: soft tissue trauma, cephalohaematoma, jaundice, hyperbilirubinaemia treated with intensive phototherapy and transitory brachial plexus injury. The major complications under study were hypoxic-ischaemic encephalopathy (HIE), intracranial or subgaleal haemorrhage, skull fracture, seizures and permanent brachial plexus injury. Other variables included in the study were: 5-min Apgar score <7, admission to the neonatal intensive care unit (NICU) and death.
We collected maternal data from antenatal and obstetric electronic records, including maternal demographic characteristics, parity, complications or diseases during pregnancy, and delivery characteristics. We obtained data for neonatal variables, such as gestational age, sex, birth weight and the Apgar score, from the hospital birth records. We obtained data for neonatal seizures and stays in the NICU from the NICU records. We defined brachial plexus injury as a flaccid paralysis of an arm at birth, with the passive range of motion being greater than the active range of motion.20 We classified brachial plexus injury as transitory when the neurologic deficit was transient, resolving before one year of age, and as permanent when the paresis was present past age 1 year. In our hospital, hyperbilirubinaemia is managed according to the NICE guidelines (Jaundice in Newborn Babies under 28 days, National Institute for Health and Care Excellence).21 Neonates with bilirubinaemia levels nearing the threshold for exchange transfusion received intensive phototherapy. The diagnosis of neonatal intracranial haemorrhage was based on brain imaging by the transfontanelar ultrasound technique. Hypoxic-ischaemic encephalopathy in a newborn was defined as a 5-min Apgar score of less than 5 and evidence of metabolic acidosis associated with one or more of the following signs of neurologic dysfunction: depression of the level of consciousness, respiratory depression, abnormality of muscle tone, disturbances of cranial nerve function and seizures (in the first week of life).22 We used the Sarnat staging system to classify HIE.23 Neonatal death was defined as death in the first 28 completed days of life (World Health Organization).
Our NICU admits neonates for intensive and intermediate care. The criteria for admission are: low birth weight (<1800g); prematurity (<34 weeks); need for cardiovascular or respiratory support (including oxygen therapy), major congenital anomalies, need for surgery, or convulsive seizures or other neurologic problems requiring neurologic monitoring, need for IV therapy, persistent hypoglycaemia despite oral feeds, hyperbilirubinaemia requiring intensive phototherapy or exchange transfusion, or need for stabilisation prior to transfer to another unit.
We analysed the data with the software SPSS version 22. We compared continuous variables using the t test and categorical variables using the χ2 test or the Fisher exact test as appropriate. We fitted multivariable logistic regression models including variables that exhibited significant differences between groups in the univariable analysis. We defined statistical significance as a P-value of less than .05.
Since the study consisted in the analysis of anonymized hospital population data, it was deemed exempt from the need to obtain approval from the Hospital Ethics Committee.
ResultsBetween January 1, 2012 and December 31, 2016, there were 12463 deliveries of newborns at 24 weeks or more of gestational age in our hospital. A total of 5991 (48%) corresponded to spontaneous vaginal deliveries, 3412 (27%) to caesarean deliveries, 2068 (17%) to vacuum-assisted vaginal deliveries and 992 (8%) to forceps-assisted deliveries.
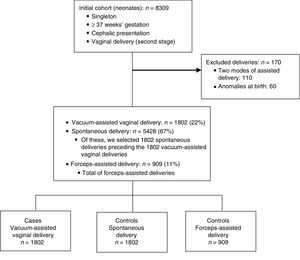
The initial cohort under study included 8309 full-term singleton neonates delivered vaginally with a cephalic presentation. After applying the exclusion criteria, the cohort included 8139 deliveries: 5428 (67%) spontaneous deliveries, 1802 (22%) vacuum-assisted vaginal deliveries and 909 (11%) forceps-assisted deliveries. The final sample included 1802 vacuum-assisted vaginal deliveries, 1802 spontaneous deliveries and 909 forceps-assisted vaginal deliveries (Fig. 1).
Compared to the spontaneous delivery group, in the vacuum-assisted delivery group mothers were more likely to be nulliparous (74% vs 43%; P<.001) (Table 1), while the newborns exhibited a predominance of the male sex (55% vs 49%; P=.001), were born at higher gestational ages (39.1 weeks vs 38.9 weeks; P<.001) and had greater birth weights (3255g vs 3193g; P<.001). We found no significant differences in the frequency of birth weight greater than 4000g and greater than 4500g between these groups (Table 1).
Main maternal and neonatal characteristics in cases and controls.
| Vacuum-assisted vaginal deliveryn=1802 | Spontaneous vaginal deliveryn=1802 | VD vs SVD | Forceps-assisted deliveryn=909 | VD vs FD | |
|---|---|---|---|---|---|
| P | P | ||||
| Maternal age, years – mean (SVD)a | 31.4 (±5) | 31.6 (±5) | 0.234 | 31.4 (±5) | 0.845 |
| Nulliparous, n (%)b | 1338 (74.0) | 782 (43) | <0.001 | 569 (71) | 0.096 |
| Maternal hypertension, n (%)b | 25 (1.4) | 34 (1.9) | 0.237 | 9 (1.1) | 0.587 |
| Gestational diabetes, n (%)b | 57 (3.2) | 73 (4.1) | 0.153 | 24 (3.0) | 0.825 |
| Preeclampsia, n (%)b | 16 (0.9) | 10 (0.6) | 0.238 | 3 (0.4) | 0.156 |
| Male sex, n (%)b | 987 (55.0) | 884 (49) | 0.001 | 435 (54) | 0.851 |
| Gestational age, weeks – mean (SD)a | 39.1 (±1) | 38.9 (±1) | <0.001 | 39.1 (±1) | 0.608 |
| Birth weight, grams – mean (SD)a | 3255 (±389) | 3193 (±405) | <0.001 | 3226 (±386) | 0.087 |
| >4000g (%) | 3.3 | 2.7 | 0.280 | 3.4 | 0.894 |
| >4500g (%) | 0.2 | 0.2 | 0.100 | 0.0 | 0.319 |
VD, vacuum-assisted vaginal delivery; FD, forceps delivery; SVD, spontaneous vaginal delivery; sd, standard deviation; bold, p value < 0.05.
The most frequent minor complication in vacuum-assisted vaginal deliveries was jaundice (14.7%), followed by soft tissue trauma (4.4%), hyperbilirubinaemia treated with intensive phototherapy (2.8%), cephalohaematoma (1.9%) and transient brachial plexus injury (0.6%).
The proportions of major complications in vacuum-assisted vaginal deliveries were 0.2% for permanent brachial plexus injury, 0.2% for seizures and 0.1% for cranial fracture, intracranial or subgaleal haemorrhage and HIE (grade 3 – severe). There were no cases of stage 1 or 2 HIE. All cases of intracranial or subgaleal haemorrhages and cases of HIE occurred in the vacuum delivery group.
The mortality rate of vacuum-assisted vaginal deliveries was 0.1% (1 per 1000), and corresponded to a total of 2 deaths. Both deaths were associated to subgaleal and intracranial haemorrhage and HIE.
In the vacuum-assisted vaginal delivery group, 0.3% of newborns had an Apgar score of less than 7, and 4.2% were admitted to the NICU.
Vacuum-assisted vaginal deliveries were associated with a significantly higher risk of minor complications compared to spontaneous deliveries, and the difference persisted after adjusting for the potential confounding variables. The adjusted odds ratio was 2.4 for soft tissue trauma (P<.001), 5.5 for cephalohematoma (P<.001), 4.4 for jaundice (P<.001), 2.1 for jaundice treated with intensive phototherapy (P<.001) and 2.1 for transient brachial plexus injury (P=.006) (Table 2).
Minor and major neonatal complications: vacuum-assisted vaginal delivery vs spontaneous delivery.
| Vacuum-assisted vaginal deliveryn=1802 | Spontaneous vaginal deliveryn=1802 | OR(95% CI) | P | Adjusted OR(95% CI)b | P | |
|---|---|---|---|---|---|---|
| Minor complications | ||||||
| Soft tissue trauma, n (%)a | 80 (4.4) | 14 (0.8) | 5.9 (3.4–10.5) | <.001 | 2.4 (1.9–2.9) | <.001 |
| Cephalohaematoma, n (%)a | 35 (1.9) | 8 (0.4) | 4.4 (2.1–9.6) | <.001 | 5.5 (3.0–9.9) | <.001 |
| Jaundice, n (%)a | 264 (14.7) | 140 (7.8) | 2.0 (1.6–2.5) | <.001 | 4.4 (1.0–9.8) | <.001 |
| Intensive phototherapy, n (%)a | 51 (2.8) | 23 (1.3) | 2.5 (1.4–3.7) | .001 | 2.1 (1.6–2.6) | <.001 |
| Transient brachial plexus injury, n (%a | 10 (0.6) | 1 (0.1) | 10.1 (1.3–78.6) | .007 | 2.1 (1.2–3.5) | .006 |
| Major complications | ||||||
| Permanent brachial plexus injury, n (%)a | 4 (0.2) | 1 (0.1) | 4.0 (0.5–35.9) | .375 | – | – |
| Cranial fracture, n (%)a | 1 (0.1) | 0 (0.0) | 1.0 (1.0–1.0) | 1.000 | – | – |
| Neonatal seizure, n (%)a | 3 (0.2) | 1 (0.1) | 3.0 (0.3–28.9) | .625 | – | – |
| IC haemorrhage, n (%)a | 2 (0.1) | 0 (0.0) | 1.0 (1.0–1.0) | .500 | – | – |
| Subgaleal haemorrhage, n (%)a | 2 (0.1) | 0 (0.0) | 1.0 (1.0–1.0) | .500 | – | – |
| HIE, n (%)a | 2 (0.1) | 0 (0.0) | 1.0 (1.0–1.0) | .125 | – | – |
| 5-min Apgar score <7, n (%)a | 5 (0.3) | 2 (0.1) | 2.5 (0.5–13.0) | .453 | – | – |
| NICU admission, n (%)a | 75 (4.2) | 40 (2.2) | 1.9 (1.3–2.8) | .001 | – | – |
| Death, n (%)a | 2 (0.1) | 0 (0.0) | 1.0 (1.0–1.0) | .500 | – | – |
HIE, hypoxic-ischaemic encephalopathy; IC, intracranial; NICU, neonatal intensive care unit; OR, odds ratio.
We found a significantly greater proportion of NICU admissions in vacuum-assisted vaginal deliveries compared to spontaneous deliveries (4.2% vs 2.2%; OR, 1.9; P=.001). The mortality rate was higher in the vacuum group, although not significantly.
When we compared maternal and neonatal characteristics we found no significant differences between vacuum and forceps-assisted deliveries (Table 1). Compared to forceps-assisted delivery, vacuum delivery was associated with a higher incidence of soft tissue trauma (4.4% vs 2.1%; OR, 2.1; P=.004), cephalohaematoma (1.9% vs 0.3%; OR, 1.4; P=.046) and jaundice (14.7% vs 11%; OR, 1.4; P=.012), but we found no differences in jaundice treated with intensive phototherapy or transient brachial plexus injury (Table 3). There was a higher frequency of major complications and death in the vacuum group, but the differences were not statistically significant (Table 3).
Minor and major neonatal complications: vacuum-assisted vaginal delivery vs forceps-assisted delivery.
| Vacuum-assisted vaginal deliveryn=1802 | Forceps-assisted deliveryn=909 | OR(95% CI) | P | |
|---|---|---|---|---|
| Minor complications | ||||
| Soft tissue trauma, n (%)a | 80 (4.4) | 17 (2.1) | 2.1 (1.3–3.6) | .004 |
| Cephalohaematoma, n (%)a | 35 (1.9) | 7 (0.3) | 2.2 (0.9–5.1) | .046 |
| Jaundice, n (%)a | 264 (14.7) | 88 (11.0) | 1.4 (1.1–1.8) | .012 |
| Intensive phototherapy, n (%)a | 51 (2.8) | 15 (1.9) | 1.5 (0.9–2.7) | .153 |
| Transient brachial plexus injury, n (%)a | 10 (0.6) | 3 (0.4) | 1.5 (0.4–5.4) | .785 |
| Major complications | ||||
| Permanent brachial plexus injury, n (%)a | 4 (0.2) | 2 (0.2) | 0.9 (0.2–4.9) | .891 |
| Cranial fracture, n (%)a | 1 (0.1) | 1 (0.1) | 0.4 (0.0–7.1) | .520 |
| Neonatal seizure, n (%)a | 3 (0.2) | 0 (0.0) | 1.0 (1.0–1.0) | .557 |
| IC haemorrhage, n (%)a | 2 (0.1) | 0 (0.0) | 1.0 (1.0–1.0) | 1.000 |
| Subgaleal haemorrhage, n (%)a | 2 (0.1) | 0 (0.0) | 1.0 (1.0–1.0) | 1.000 |
| HIE, n (%)a | 2 (0.1) | 0 (0.0) | 1.0 (1.0–1.0) | .500 |
| 5-min Apgar score <7, n (%)a | 5 (0.3) | 1 (0.1) | 2.2 (0.3–19.1) | .673 |
| NICU admission, n (%)a | 75 (4.2) | 22 (2.8) | 1.5 (0.9–2.5) | .079 |
| Death, n (%)a | 2 (0.1) | 0 (0.0) | 1.0 (1.0–1.0) | 1.000 |
HIE, hypoxic-ischaemic encephalopathy; IC, intracranial; NICU, neonatal intensive care unit; OR, odds ratio.
The rates of vacuum-assisted vaginal deliveries are difficult to compare, as different studies use different methodologies and medical practices vary between countries. In our study, we found a vacuum delivery proportion of 22% in term singleton neonates, compared to 11% in a similar study.1
Studies comparing neonatal and maternal morbidity by modes of delivery point to a multiplicity of risk factors, such as birth weight,5,16,25 which could result in bias. In our study, we adjusted the multivariate analysis for all the maternal and neonatal characteristics that differed between groups and that could act as confounders, and the differences persisted for minor complications. When it came to birth weight, we found a difference of 62g in the mean birth weight between the vacuum and spontaneous groups. In our opinion, this weight difference should not be considered clinically relevant. There was also no difference in the number of neonates with macrosomia. Thus, we concluded that birth weight did not seem to have an impact on the outcomes of our study. The frequency of male sex was also higher in the vacuum group compared to the spontaneous group, but we could not establish a direct correlation between poor outcomes in the vacuum group and the higher frequency of males. However, a recent study26 concluded that the pulmonary circulation and the lungs are more mature in female newborns, who are therefore better equipped to face the challenges involved in the transition from foetal to neonatal life and postnatal adaptation.
Our study revealed higher rates of all minor complications in neonates born by vacuum-assisted vaginal delivery compare to those born by spontaneous vaginal delivery or by forceps-assisted delivery, except for jaundice treated with intensive phototherapy and transient brachial plexus injury in the forceps group.
Cephalohaematoma has been associated with vacuum-assisted vaginal delivery compared to forceps delivery.7–9 In our cohort, the incidence of cephalohaematoma was lower than previously reported (1.9% vs 15%),15,24–27 which may be explained by underreporting. In most cases, cephalohaematoma does not require treatment, although the reabsorption of the cephalohaematoma may cause jaundice.9,2–25 Another possible cause of jaundice is the reabsorption of bruising. The higher incidence of soft tissue trauma and cephalohaematoma could explain the higher incidence of jaundice in the vacuum group compared to the other modes of vaginal delivery. However, the incidence of scalp abrasions and lacerations was also lower compared to those reported in other studies (4.4% vs 10%).15 Once more, given that these complications are transient and of no clinical relevance, they might be underreported and thus explain these discrepancies.
In our study, the incidence of obstetric brachial plexus injuries (4.7 per 1000) was similar to the incidence described in the literature (0.38-5.8 per 1000).28 There are identified risk factors for brachial plexus injuries, such as macrosomia, shoulder dystocia, vacuum and forceps-assisted delivery and excessive maternal weight gain.29 Furthermore, some studies report that a birth weight greater than 4000g is independently associated with poor neonatal outcomes after vacuum-assisted vaginal delivery.5,25 However, in our study, the increased risk of transient brachial plexus injury in vacuum-assisted vaginal deliveries compared to spontaneous deliveries was independent of birth weight.
The incidence of major complications was higher in the vacuum group compared to the forceps and spontaneous delivery groups, but the difference was not significant. Since major complications were rare events, a larger sample would be required to demonstrate a significant difference.
The most serious major complications (subgaleal and intracranial haemorrhages and hypoxic-ischaemic encephalopathy) and the two deaths were associated, and all occurred in the vacuum group. The intracranial haemorrhage incidence for vacuum-assisted vaginal deliveries was 1.1 per 1000. The incidence varies in the literature, with a study reporting an incidence of traumatic intracranial haemorrhage of 0.6 per 1000 in neonates born to term by vacuum-assisted vaginal delivery5 and another study reporting higher incidences (8.8 per 1000).16 The location of intracranial haemorrhage may be subdural, subarachnoid, intraparenchymal or intraventricular. There are many risk factors for intracranial haemorrhage (birth asphyxia, prematurity, bleeding diathesis, infection, vascular anomalies), but subdural haemorrhage is almost always a result of birth trauma.30 We found no risk factors for infection and vascular anomalies were not identified in the transcranial Doppler ultrasound. In our NICU, neonates with major neurologic symptoms usually undergo a head magnetic resonance imaging (MRI) scan. However, MRI was not performed in the 2 cases of brain haemorrhage, as these patients were unstable and died in the first days of life. This fact is particularly important, as some haemorrhages cannot be detected by ultrasound. These cases were also not evaluated for bleeding diathesis. Subgaleal haemorrhage is a rare but potentially life-threatening condition, and it has been associated with vacuum-assisted vaginal delivery.15,31,32 It results from the rupture of emissary veins and their subsequent bleeding into the potential space between the epicranial aponeurosis and the periosteum of cranial bones. The incidence of subgaleal haematoma in vacuum-assisted vaginal deliveries was 1.1 per 1000, whereas in a previous study conducted in Australia it was 7.6 per 1000.32 Two reasons could explain this discrepancy, including methodological differences, such as the inclusion of all births, and the underreporting and misdiagnosis of mild cases. Once again, bleeding diathesis was not ruled out in these cases. The incidence of neonatal hypoxic-ischaemic encephalopathy in vacuum-assisted vaginal deliveries was also lower compared to previous studies with similar inclusion criteria1 (1.1 per 1000 vs 4.7 per 1000). Unlike stage 2 and 3, stage 1 of HIE may be easily underdiagnosed since the symptoms are subtle and may be present in other conditions. We found no stage 1 cases in our study over a 5-year period, which could also have been due to misdiagnosis or underreporting.
The overall mortality associated with the two methods of assisted vaginal delivery was 7 per 10000. Other authors have reported a lower risk of neonatal death secondary to intracranial haemorrhage (3–4 per 10000) in association with assisted vaginal delivery with either of these two instruments (vacuum cup or forceps).1
NICU admission was more frequent in assisted vaginal deliveries compared to spontaneous deliveries. The evidence on this aspect is inconsistent, with some studies concluding that newborns delivered by any type of operative approach are at increased risk of NICU admission,33 other concluding that the use of forceps is associated with higher rates of NICU admission compared to vacuum delivery,34 and yet others claiming that NICU admission should not be considered a variable reflecting neonatal morbidity.35 However, we considered NICU admission a relevant variable in our study, since it involves separation from mother, which has been associated with difficulties in breastfeeding.
We acknowledge several potential limitations to the present study. The retrospective design is a potential source of bias. However, the data for most of the variables under study are collected and recorded prospectively and routinely, and reported annually. This is a relevant point, especially when it comes to retrospective studies. In addition, the large size of our cohort should limit this effect. However, the sample size was still small for rare complications.
Our study, as occurs in most studies on the outcomes of different modes of delivery and neonatal outcomes, is limited by the inherent difficulty of separating the indication for the mode of delivery from the mode itself. Although vacuum-assisted vaginal delivery is associated with minor and major neonatal complications in many studies, it is not clear whether the complications are caused by the vacuum extraction or the vacuum extraction is needed because of the complication (abnormal foetal heart rate, foetal dystocia, prolonged labour). So, we cannot assert that operative vaginal delivery is an independent risk factor for higher rates of morbidity. We did not study some variables that could interfere with the outcome, such as maternal comorbidities, the indication for vacuum extraction, the foetal presentation and the number of pulls, which is also a limitation of our study. The choice between the use of forceps and vacuum devices depends essentially on the position of the foetal head in the birth canal and on maternal variables also related to the birth canal. We did not analyse these variables, but we think they are not a source of bias, as the choice of technique has more to do with the conditions of applicability than with the foetal presentation.
However, there are several strengths to our study that distinguish it from previous studies on the subject. All neonates were delivered in a single tertiary centre with a high volume of patients and a standardised management of all pregnant women. All assisted vaginal deliveries were performed by an obstetrician or a obstetric resident under supervision. For each case of vacuum-assisted vaginal delivery, we included a case of forceps-assisted delivery and a case of spontaneous delivery managed by the same team, which we expected would minimise bias.
For fully-dilated women with singleton pregnancies come to term, vacuum-assisted vaginal delivery is associated with a higher incidence of minor neonatal complications compared to forceps-assisted or spontaneous delivery. It is known that spontaneous delivery is the optimal mode of delivery for both mother and newborn when the necessary obstetric and neonatal conditions are met. However, vacuum-assisted vaginal delivery should be performed if there is a formal indication for it. Vacuum cups should be used by either obstetricians or residents under the guidance of a specialist.
The potential complications of vacuum-assisted delivery warrants closer monitoring of these neonates in the first 12–24h by neonatologists, who should always be informed about the use of these devices.
Conflict of interestsThe authors declare that they have no conflicts of interest.
Please cite this article as: Ferraz A, Nunes F, Resende C, Almeida MC, Taborda A. Complicaciones neonatales a corto plazo de los partos por ventosa. Estudio caso-control. An Pediatr (Barc). 2019;91:378–385.