Impetigo is one of the most common infectious diseases in children.1 The most frequent causative agents are Staphylococcus aureus and Streptococcus pyogenes. Cutaneous lesions resulting from self-inoculation and its highly contagious nature facilitate the dissemination of bacteria among patients and close contacts.2 It is a clinical diagnosis, and conventional treatment for mild cases with localized lesions consists of topical application of mupirocin or fusidic acid.3 When lesions are numerous, disseminated or refractory to topical agents, the recommended treatment is oral cefadroxil or cloxacillin.4
The aim of our study was to analyze resistance patterns in isolated S. aureus strains at both the phenotypic and genotypic levels as well as virulence factors. We also aimed to describe the clinical presentation of the impetigo outbreak and the outcomes in affected patients, as well as the initial treatment prescribed and the frequency with which it required modification.
Between June and September 2023, we observed an increase in the number of impetigo cases with empirical treatment failure among the skin samples sent to the microbiology laboratory. The review of the microbiological results called for an official outbreak investigation by the public health authorities. Twelve S. aureus isolates were submitted to the Staphylococcal Infection Surveillance Program of the Centro Nacional de Microbiología (National Microbiology Center of Spain) for molecular analysis by means of whole-genome sequencing (WSG). For two of the strains, the number of reads was insufficient for WGS, so instead they were studied by RT-PCR for toxin identification and PCR for spa-typing.
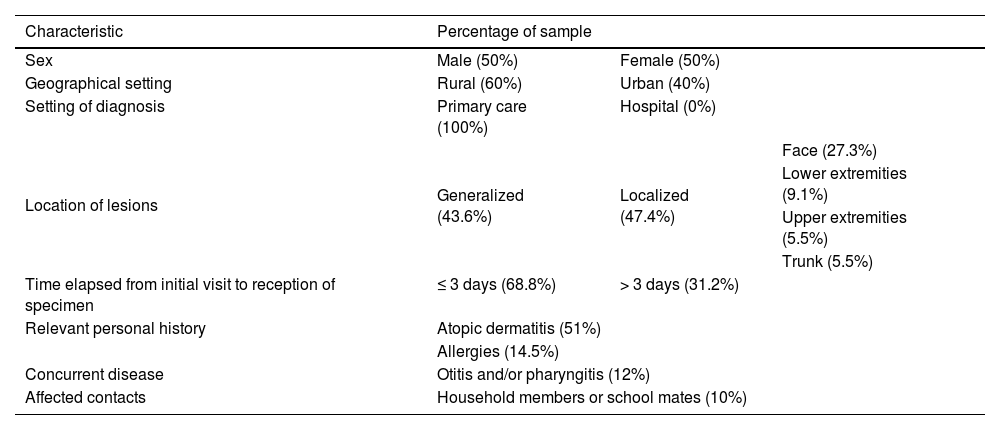
A total of 48 samples were collected, and Table 1 summarizes their characteristics. The median patient age was 6.8 years (6mo–13 years). In 68.2% of cases, the sample was collected in the initial visit, and, in the remaining cases, samples were collected later due to clinical worsening, inadequate initial clinical suspicion or re-evaluation of the patient by the pediatrician. The mean duration of symptoms was 11 days, and seven patients experienced recurrence one month after resolution of the initial episode. Two patients required hospital admission: one due to extensive lesions refractory to outpatient treatment and another due to eczema herpeticum. In addition, two patients developed dyshidrotic eczema at the location of the lesions.
Sample characteristics.
| Characteristic | Percentage of sample | ||
|---|---|---|---|
| Sex | Male (50%) | Female (50%) | |
| Geographical setting | Rural (60%) | Urban (40%) | |
| Setting of diagnosis | Primary care (100%) | Hospital (0%) | |
| Location of lesions | Generalized (43.6%) | Localized (47.4%) | Face (27.3%) |
| Lower extremities (9.1%) | |||
| Upper extremities (5.5%) | |||
| Trunk (5.5%) | |||
| Time elapsed from initial visit to reception of specimen | ≤ 3 days (68.8%) | > 3 days (31.2%) | |
| Relevant personal history | Atopic dermatitis (51%) | ||
| Allergies (14.5%) | |||
| Concurrent disease | Otitis and/or pharyngitis (12%) | ||
| Affected contacts | Household members or school mates (10%) | ||
Staphylococcus aureus was isolated from every sample, with additional isolation of Streptococcus dysgalactiae in one sample and S. pyogenes in three.
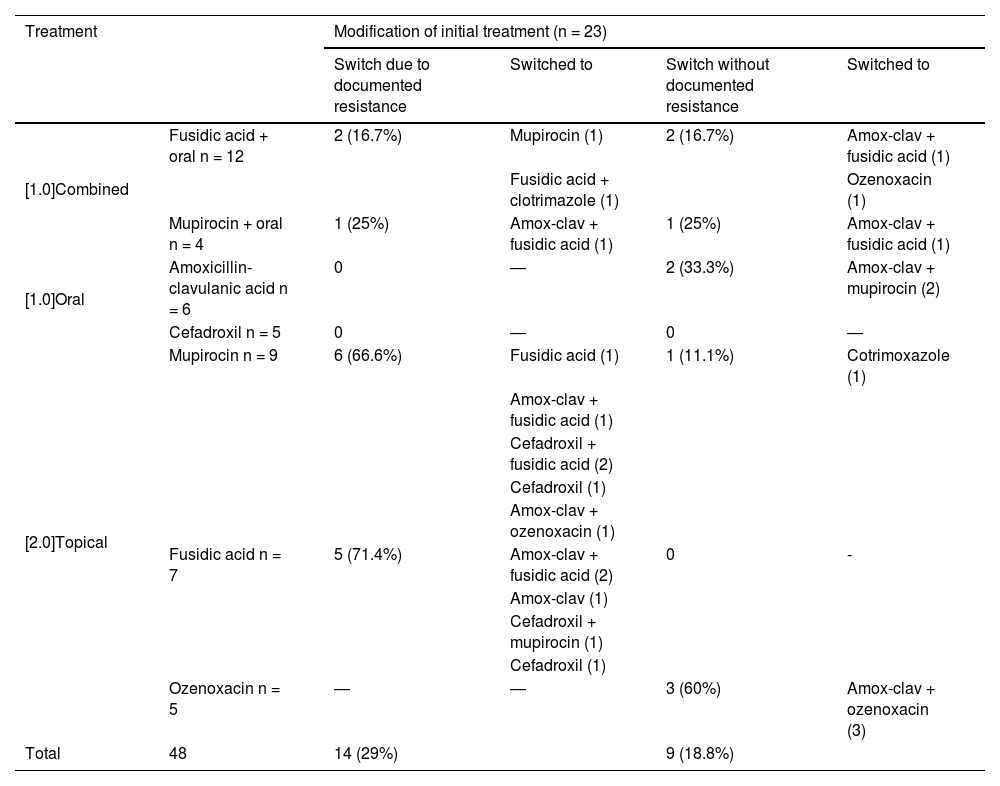
Table 2 presents the treatments initially prescribed and the changes made to the initial treatment. Changes were made in 48% of cases by adding or switching drugs. Of the 32 initial treatments that included fusidic acid or mupirocin, 18 required modification, which in 17 cases consisted in the addition or switch to an oral beta-lactam antibiotic. Of all S. aureus isolates, 76% were resistant to fusidic acid, 86% to mupirocin and 72% to both. In the subset of cases in which sample collection took place more than two days after treatment initiation, strains resistant to fusidic acid or mupirocin were isolated from 86.6% of the samples. Antimicrobial susceptibility testing was not performed for ozenoxacin due to the lack of established cut-off points for this drug, but quinolones exhibited in vitro activity against all isolates. One isolate was resistant to cotrimoxazole and another to clindamycin.
Prescribed initial treatment and subsequent changes.
| Treatment | Modification of initial treatment (n = 23) | ||||
|---|---|---|---|---|---|
| Switch due to documented resistance | Switched to | Switch without documented resistance | Switched to | ||
| [1.0]Combined | Fusidic acid + oral n = 12 | 2 (16.7%) | Mupirocin (1) | 2 (16.7%) | Amox-clav + fusidic acid (1) |
| Fusidic acid + clotrimazole (1) | Ozenoxacin (1) | ||||
| Mupirocin + oral n = 4 | 1 (25%) | Amox-clav + fusidic acid (1) | 1 (25%) | Amox-clav + fusidic acid (1) | |
| [1.0]Oral | Amoxicillin-clavulanic acid n = 6 | 0 | ― | 2 (33.3%) | Amox-clav + mupirocin (2) |
| Cefadroxil n = 5 | 0 | ― | 0 | ― | |
| [2.0]Topical | Mupirocin n = 9 | 6 (66.6%) | Fusidic acid (1) | 1 (11.1%) | Cotrimoxazole (1) |
| Amox-clav + fusidic acid (1) | |||||
| Cefadroxil + fusidic acid (2) | |||||
| Cefadroxil (1) | |||||
| Amox-clav + ozenoxacin (1) | |||||
| Fusidic acid n = 7 | 5 (71.4%) | Amox-clav + fusidic acid (2) | 0 | - | |
| Amox-clav (1) | |||||
| Cefadroxil + mupirocin (1) | |||||
| Cefadroxil (1) | |||||
| Ozenoxacin n = 5 | ― | ― | 3 (60%) | Amox-clav + ozenoxacin (3) | |
| Total | 48 | 14 (29%) | 9 (18.8%) | ||
Of the five patients initially treated with ozenoxacin, three required subsequent addition of oral treatment due to extensive involvement. There were no documented cases of resistance to oral treatments.
All strains that were sequenced for which there were sufficient reads (n = 10) carried the eta and etb genes that encode exfoliative toxins ETA and ETB and belonged to clonal complex CC121.
All isolates carried the blaZ gene, which causes penicillin resistance. Most (8/10) had carried the mupA gene in a plasmid, which confers high-level resistance to mupirocin.
At the global level, approximately 5% of S. aureus strains produce exfoliative toxins, all of which are associated with clonal complex CC121 and are usually sensitive to methicillin.5 The sequencing results for the submitted strains supported our results, as they belonged to clonal complex CC121 and most carried genes conferring resistance to mupirocin and fusidic acid. The presence of genes that encode exfoliative toxins ETA and ETB explains the development of skin lesions and the effective dissemination of the pathogen. This constitutes a shift in relation to the circulating strains in the past few years that is relevant to the prescribing of empirical therapy.
Modifications to initial treatment were necessary in more than half the cases, with higher proportions of treatment switches or modification among patients who started treatment with mupirocin or fusidic acid. Oral treatment needed to be added in more than half of the cases in which the initial management was limited to topical treatment, including three patients treated with ozenoxacin. Combination therapy was required due to worsening and/or extensive cutaneous involvement.
In light of this epidemiological context, pediatricians in our health care area modified their approach to the management of impetigo, collecting samples for culture with greater frequency and considering the use of ozenoxacin in place of the customary topical antibiotics.
In conclusion, knowledge of local antimicrobial resistance patterns in strains that cause impetigo is important to guide treatment. We recommend active surveillance of the detection and dissemination of S. aureus strains causing skin and soft tissue infections in the community, as well as characterization of the predominant circulating lineages at regular intervals.
FundingThis research project did not receive specific financial support from funding agencies in the public, private or not-for-profit sectors.
Previous meeting: this study was presented as an e-poster at the XXVII Congress of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica; May 30–June 1, 2024; Zaragoza, Spain.