Rabies is a zoonotic viral disease found all over the world. Dogs are the source of infection in over 99% of human cases.
Rabies is caused by an RNA virus in the family Rhabdoviridae family, genus Lyssavirus, found in the saliva of infected mammals. The virus is transmitted to humans through bites from such animals, and the risk of contracting the disease ranges between 5% and 80% depending on the intensity of the contact and the amount of inoculated pathogen.
In Europe, the epidemiology of rabies has changed in recent years due to extensive establishment of the virus in bats, the re-emergence of rabies in foxes, and the import of rabid dogs from North Africa.1
Spain had been free of terrestrial rabies since 1978. In June of 2013 a case of rabies was confirmed in a dog that had come from Morocco. A level 1 rabies alert was declared, and a restricted area for interventions set up across several autonomous communities (Catalonia, Castilla-La Mancha and Madrid).2,3 The Hospital Carlos III was designated as the reference centre for patients that had had animal contacts within the restricted area.
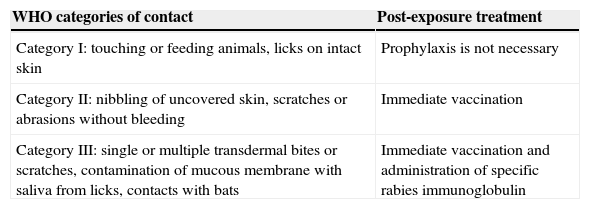
We performed a retrospective, observational descriptive study based on the review of medical records from suspected paediatric cases during the rabies alert period. We collected data on epidemiology variables, the source of transmission, the contact category as defined by the WHO classification4 (Table 1) and the subsequent interventions.
Categories of contact defined by the WHO.
| WHO categories of contact | Post-exposure treatment |
|---|---|
| Category I: touching or feeding animals, licks on intact skin | Prophylaxis is not necessary |
| Category II: nibbling of uncovered skin, scratches or abrasions without bleeding | Immediate vaccination |
| Category III: single or multiple transdermal bites or scratches, contamination of mucous membrane with saliva from licks, contacts with bats | Immediate vaccination and administration of specific rabies immunoglobulin |
During the rabies alert, 29 suspected cases were seen at the hospital; 9 of them (31%) occurred in children. The mean age of these patients was 9.8 years, and 66.7% of them were male. Eight cases involved dog bites, and one patient had been bitten by a rat. All patients had their wounds cleaned and were given amoxicillin-clavulanate for post-exposure prophylaxis. Five bites were classified as category II and three bites as category III as defined by the WHO, so these patients were initially considered candidates for post-exposure prophylaxis.
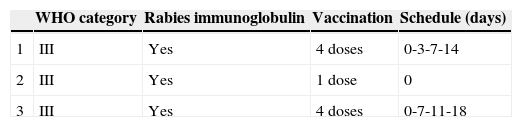
However, five of the children had been bitten out of the restricted area, and one by a vaccinated dog. Thus, post-exposure prophylaxis was only indicated in three children, and was completed in two of them. Prophylactic treatment was discontinued in the third child once the source (a dog) was identified and its vaccinated status verified. The interventions performed on these children are specified in Table 2.
In one patient, the vaccination schedule consisted of doses at days 0, 7, 11, and 18, with a delay between the first and second dose due to the patient's condition.
During the alert, no human rabies cases were detected, and the index dog had the only confirmed case. Interventions adhered to the established protocols with category I contacts, not requiring prophylactic treatment. Category II contacts require immediate vaccination, and immediate vaccination combined with human rabies immunoglobulin is recommended for category III contacts. All wounds have to be washed with soap and water for 15min. Only two vaccine preparations are marketed in Spain (Rabipur® and Merieux®), both of which have an efficacy of 100% for post-exposure prophylaxis combined with the other established measures.1,4,5
The schedule of post-exposure prophylaxis recommended for unvaccinated individuals consists of 4 vaccine doses delivered intramuscularly. The first dose should be given as soon as possible, and the date it is administered is considered to be day 0 in the series. The remaining doses should be administered on days 3, 7 and 14. The full dose of immunoglobulin should be injected in the edges of the wound, and if this were not possible any remaining volume should be injected intramuscularly in a site distant from vaccine administration.6
None of the patients had side effects from the vaccine or the rabies immunoglobulin, which confirms their safety profiles.
Since 1979, all cases of rabies due to EBLV1 reported in Spain were infections in bats. The presence of this virus was also detected in the course of two field studies on bats between 1989 and 2004, which confirms that aerial rabies is endemic in Spain.
Still, it is the presence of terrestrial rabies in Europe and more importantly Morocco that poses a constant threat of imported cases.
On December 23, 2013, six months after its declaration, the rabies alert was lifted and Spain reverted to alert level 0 (no animal rabies cases).
The interdisciplinary coordination of the action teams, in which a Centro de Referencia Pediátrico para Alertas Sanitarias Infecciosas (Paediatric Reference Centre for Infectious Health Alerts) played an essential part, was key to preventing this episode from leading to an outbreak during the alert.
Please cite this article as: García García E, García López Hortelano M, Mellado Peña M. Alerta de rabia terrestre en España en 2013. Actuación pediátrica. An Pediatr (Barc). 2015;82:100–101.