Heart failure (HF) in pediatric hypertrophic cardiomyopathy (HCM) is usually secondary to left ventricular outflow tract obstruction (LVOTO) with preserved left ventricular ejection fraction (LVEF).
However, patients with non-obstructive HCM may develop HF due to diastolic or systolic dysfunction (burned-out or end-stage HCM). These cases pose a challenge, as no specific treatment is currently available for it.1
Sodium-glucose cotransporter 2 (SGLT2) inhibitors have been found to have cardioprotective effects and improve HF outcomes and are recommended for HF of any functional classification in adults,2 with additional evidence of their usefulness for management of non-obstructive HCM in particular.1,3 It also seems to be a safe and effective group of drugs for management of pediatric HF, but the evidence is still scarce.4–6
We describe the use of SGLT2 inhibitors as adjuvant therapy in three adolescents with HF due to non-obstructive HCM, highlighting its safety and the observed benefits.
- none-
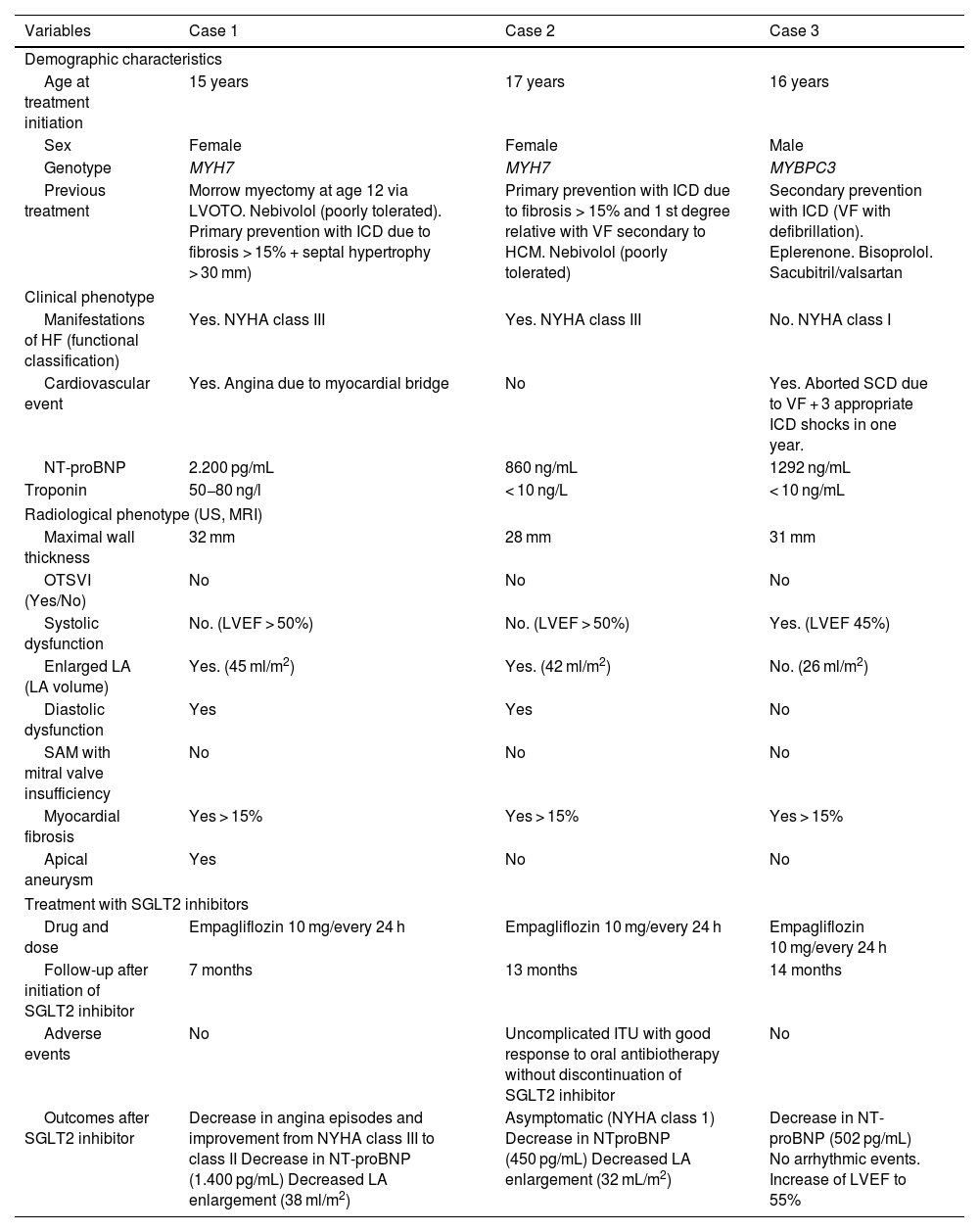
Case 1: Female adolescent aged 15 years with a history of Morrow septal myectomy and diastolic dysfunction with NYHA class III heart failure. The patient started treatment with empagliflozin (10 mg/24 h), with clinical improvement to NYHA class II with a reduction in atrial dilation and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels at 6 months of treatment, without adverse events.
- none-
Case 2: Female adolescent aged 17 years with diastolic dysfunction and NYHA class III heart failure. After 9 months of treatment with empagliflozin (10 mg/24 h), she improved to NYHA class I with normalization of the left atrium, a decrease in NT-proBNP and an uncomplicated urinary tract infection as an adverse event.
- none-
Case 3: Male adolescent aged 16 years with HCM and an implantable cardioverter-defibrillator, who had no symptoms of HF but received frequent shocks and had a LVEF of 45%. After 12 months of treatment with 10 mg of empagliflozin every 24 h, the LVEF increased to 55% and ventricular arrhythmias had ceased, with no adverse events.
Table 1 provides more detailed clinical and testing information.
Demographic, clinical and radiological characteristics of patients before initiation of SGLT2 inhibitor treatment and subsequent outcomes.
| Variables | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Demographic characteristics | |||
| Age at treatment initiation | 15 years | 17 years | 16 years |
| Sex | Female | Female | Male |
| Genotype | MYH7 | MYH7 | MYBPC3 |
| Previous treatment | Morrow myectomy at age 12 via LVOTO. Nebivolol (poorly tolerated). Primary prevention with ICD due to fibrosis > 15% + septal hypertrophy > 30 mm) | Primary prevention with ICD due to fibrosis > 15% and 1 st degree relative with VF secondary to HCM. Nebivolol (poorly tolerated) | Secondary prevention with ICD (VF with defibrillation). Eplerenone. Bisoprolol. Sacubitril/valsartan |
| Clinical phenotype | |||
| Manifestations of HF (functional classification) | Yes. NYHA class III | Yes. NYHA class III | No. NYHA class I |
| Cardiovascular event | Yes. Angina due to myocardial bridge | No | Yes. Aborted SCD due to VF + 3 appropriate ICD shocks in one year. |
| NT-proBNP | 2.200 pg/mL | 860 ng/mL | 1292 ng/mL |
| Troponin | 50−80 ng/l | < 10 ng/L | < 10 ng/mL |
| Radiological phenotype (US, MRI) | |||
| Maximal wall thickness | 32 mm | 28 mm | 31 mm |
| OTSVI (Yes/No) | No | No | No |
| Systolic dysfunction | No. (LVEF > 50%) | No. (LVEF > 50%) | Yes. (LVEF 45%) |
| Enlarged LA (LA volume) | Yes. (45 ml/m2) | Yes. (42 ml/m2) | No. (26 ml/m2) |
| Diastolic dysfunction | Yes | Yes | No |
| SAM with mitral valve insufficiency | No | No | No |
| Myocardial fibrosis | Yes > 15% | Yes > 15% | Yes > 15% |
| Apical aneurysm | Yes | No | No |
| Treatment with SGLT2 inhibitors | |||
| Drug and dose | Empagliflozin 10 mg/every 24 h | Empagliflozin 10 mg/every 24 h | Empagliflozin 10 mg/every 24 h |
| Follow-up after initiation of SGLT2 inhibitor | 7 months | 13 months | 14 months |
| Adverse events | No | Uncomplicated ITU with good response to oral antibiotherapy without discontinuation of SGLT2 inhibitor | No |
| Outcomes after SGLT2 inhibitor | Decrease in angina episodes and improvement from NYHA class III to class II Decrease in NT-proBNP (1.400 pg/mL) Decreased LA enlargement (38 ml/m2) | Asymptomatic (NYHA class 1) Decrease in NTproBNP (450 pg/mL) Decreased LA enlargement (32 mL/m2) | Decrease in NT-proBNP (502 pg/mL) No arrhythmic events. Increase of LVEF to 55% |
HCM, hypertrophic cardiomyopathy; HF, heart failure; ICD, implantable cardioverter-defibrillator; LA, left atrium; LVEF, left ventricular ejection fraction; LVOTO, left ventricular outflow tract obstruction; MRI, magnetic resonance imaging; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PH, pulmonary hypertension; SAM, systolic anterior motion; SCD, sudden cardiac death; SGLT2: sodium-glucose cotransporter 2; US, ultrasound; UTI, urinary tract infection; VF, ventricular fibrillation.
Our experience adds to the emerging evidence that supports the use of SGLT2 inhibitors as a safe and effective adjuvant treatment for pediatric HF, particularly in patients with non-obstructive HCM.
A study in a cohort of 38 patients (median age of 12 years) with HF (NYHA classes 2–4) secondary to dilated cardiomyopathy (68%), single ventricle (18%) and diastolic dysfunction (10%) who received adjuvant treatment with dapagliflozin found significant improvement in both the LVEF and natriuretic peptide levels, and 16% of the patients developed uncomplicated urinary tract infections4 during the 130-day follow-up. A retrospective study that included 14 pediatric patients (median age, 14 years) with Fontan circulation treated with SGLT2 inhibitors with a median follow-up from initiation of four months found improvements in ejection fraction and a decrease in natriuretic peptide levels, in the absence of severe adverse events5 with the exception of case of significant diuresis and transient acute kidney injury. On the other hand, a systematic review of five studies concluded that SGLT2 inhibitors are safe and effective in children with HF and other diseases.6 Lastly, a study of 28 661 adults with HCM treated with SGLT2 inhibitors found significant reductions in mortality (OR, 0.24; P < .01) and hospital admissions (OR, 0.69; P = .01), in addition to a decreased incidence of cardiovascular events (OR, 0.63; P < .01), opening the possibility of extrapolating these beneficial effects to adolescents with non-obstructive HCM due to the greater physiological similarity of this age group with adults compared to children.3
The outcomes in our three patients were consistent with the previous evidence, particularly the clear subjective clinical improvement in cases 1 and 2, with reduction and resolution of dyspnea on exertion and accompanied by reductions in atrial dilation and NT-proBNP levels, and, in case 3, improvement of LVEF and resolution of ventricular arrhythmias. Also in agreement with the literature, the patients tolerated the treatment well, with the exception of an uncomplicated urinary tract infection in case 2.
With respect to the choice of SGLT2 inhibitor, we selected empagliflozin on account of the recent evidence suggesting a greater reduction in hospital admission due to HF and, theoretically, having the highest sensitivity for SGLT2 receptors within its class, although other drugs of the same class could be equally useful, given the lack of specific pediatric studies.
Despite these promising observations, there are limitations to our findings. The small sample size, the lack of a control group and not having performed objective tests such as ergospirometry to assess functional changes preclude the generalization of the results. Furthermore, the follow-up period did not allow for the evaluation of long-term clinical benefits and safety. However, the benefit observed in our patients highlights the need for further research into the potential of SGLT2 inhibitors as a safe and effective novel therapeutic option for adolescents with non-obstructive HCM, who currently have unmet treatment needs.
Meeting presentation: poster presentation at the 15th National Congress of the Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas; November 15, 2024; Murcia, Spain.