Oropharyngeal dysphagia (DOF) without proper evaluation can be underdiagnosed in certain groups of the pediatricpopulation. Meeting the needs of these patients can lead to an overload of their caregivers.
ObjectivesTo describe the epidemiological and clinical characteristics of the patients evaluated after starting a monographic DOF clinic (C-DOF) and study whether there are changes at the nutritional level, as well as the burden and impact that caregivers find on quality of life related to health (HRQL).
Material and methodsDescriptive observational study of patients evaluated in a C-DOF from its start-up. To evaluate HRQoL, an ad hoc survey adapted from the Swallowing Quality of Life Questionnaire of the adult population was designed.
Results103 patients were evaluated, 85.4% presenting some neurological disease. A videofluoroscopic study was performed in 51 patients (49.5%), reporting combined alterations in both the oral and pharyngeal phases in 64.7% of them. There was a directly proportional correlation between the severity of the DOF and the presence of aspirations, as well as with the patient's motor impairment. Regarding the anthropometric evaluation, there was a trend towards improvement in weight z-score (+0.14 SD), height (+0.17 SD) and BMI (+0.16 SD). Out of 46.2% of the caregivers reported that the DOF problem interfered negatively in the basic activities of daily life. The increase in HRQOL, after the evaluation in the monographic DOF clinic, was statistically significant overall.
ConclusionsThe monographic DOF clinic provided specialized care, impacting positively at the nutritional status of patients, as well as perceived changes in HRQOL, with a probable impact on caregivers.
La disfagia orofaríngea (DOF) puede resultar infradiagnosticada en determinados grupos de población pediátrica. Atender las necesidades de estos pacientes puede derivar en una sobrecarga de sus cuidadores.
ObjetivosDescribir las características epidemiológicas y clínicas de los pacientes evaluados tras la creación de una consulta monográfica de DOF (C-DOF), estudiar cambios a nivel nutricional, así como la repercusión en la calidad de vida relacionada con la salud (CVRS) de los cuidadores.
Material y métodosEstudio observacional descriptivo de los pacientes evaluados en una C-DOF desde su puesta en marcha. Para evaluar la CVRS, se diseña una encuesta ad hoc adaptada del Swallowing Quality of Life Questionnaire de población adulta.
ResultadosSe evaluaron 103 pacientes (85,4% con patología neurológica de base). Se realizó estudio videofluoroscópico a 51 pacientes (49,5%), reportando alteraciones combinadas de fase oral y faríngea un 64,7%. Existió una correlación directamente proporcional entre la gravedad de la DOF y la presencia de aspiraciones, así como con la afectación motora del paciente. En cuanto a la evaluación antropométrica, se observó mejoría en z-score de peso (+0,14 DE), de talla (+ 0,17 DE) y de IMC (+ 0,16 DE). El 46,2% de los cuidadores refirieron que la DOF interfiere negativamente en las actividades básicas de la vida diaria. El incremento en calidad de vida, tras la evaluación en la C-DOF, de forma global resultó estadísticamente significativo.
ConclusionesLa C-DOF proporciona una atención especializada, repercute positivamente en el estado nutricional de los pacientes, así como en los cambios percibidos en la CVRS, con probable impacto en los cuidadores.
Oropharyngeal dysphagia (OPD) is a prevalent symptom in certain paediatric population groups,1 especially in neurological patients. The incidence of OPD in paediatrics is estimated at 1%2 and eating disorders at 25%–45%, although the overall incidence of OPD is increasing3 and is much higher in at-risk populations such as those with cerebral palsy (CP), the most common neurological condition associated with dysphagia, or neurodevelopmental disorders.4 However, it is often underdiagnosed and can substantially affect the child’s health and future development.
In paediatric contexts it is difficult to standardise objective non-observer-dependent dysphagia screening methods. Dysphagia severity scales are instruments that aim to provide an assessment of patients and are useful both for individualising the approach and for evaluating the efficacy of the rehabilitative treatment. Among the most commonly used are the Dysphagia Outcome and Severity Scale (DOSS),5 the Food Intake Level Scale (FIS)6 and the Functional Oral Intake Scale (FOIS),7 all designed for the adult population. The FOIS offers an overall functional view of the severity of the dysphagia and also serves to identify changes in oral feeding over time (graded in 7 levels). Although it has been used in paediatric patients,8 versions adapted for infants and children have recently been developed,9,10 simplifying it to 5 levels. In CP the Eating and Drinking Ability Classification System (EDACS) scale11 was developed to provide a description of people’s eating abilities, according to their possible limitations in safety and efficiency.
The objectives of this study were to assess the nutritional status and clinical features of patients with dysphagia receiving attention at a specialised paediatric OPD clinic and their subsequent progress, and also to estimate the impact on the health-related quality of life of their caregivers.
Materials and methodsWe conducted an observational descriptive study of children evaluated at the specialised paediatric OPD clinic during the period between March 2019 and March 2020.
We analysed the following variables: age, underlying disease, source of and reasons for referral to the dysphagia clinic, nutritional evaluation with anthropometry, classification of motor impairment using the Gross Motor Function Classification System (GMFCS) scale,12 functionality of oral intake according to the FOIS (versions adapted for children up to the age of 7 years were used),9,10 videofluoroscopic swallow study (VFSS) for diagnosis and if the presence or absence of signs of dysphagia (abnormalities in the oral and/or pharyngeal phase) is observed, presence of aspiration and/or penetration, type of aspiration (silent or with coughing), assessment of the severity of the dysphagia according to the EDACS scale,11 specific speech therapy rehabilitation at an outpatient clinic, the prescription and indications for external feeding devices and anthropometric growth during the follow-up.
For the anthropometric study, we used the World Health Organization’s growth standards for children under 5 years13 and Spanish growth studies14 for the rest, thus following the 2017 recommendation of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) to assess growth based on a standardised reference population.15 In those patients whose stature could not be measured properly in a standing position, it was estimated using Stevenson’s formula16 based on tibial length (TL): stature = (TL × 3.26) + 30.8 cm.
The VFSS was performed with a Siemens Luminos Fusion fluoroscopy machine. The pulsed fluoroscopy technique was used, obtaining real-time moving images with a frequency of 15 pulses per second (pps).
The scope time was controlled by a radiologist. Collimators were used, to minimise scatter radiation, as well as automatic exposure dose control. The table was placed in a vertical position and the patients were positioned sitting on an adapted chair (at 90 °) or with a child restraint seat and three-point anchor system if necessary, with the X-ray tube in a lateral projection and the focus centred on the patient’s oropharynx. The whole procedure was performed with continuous monitoring by pulse oximetry. Visipaque water-soluble contrast medium was administered, in various volumes and textures (thin liquid, nectar-thick and pudding-thick) prepared beforehand by the medical team. If there was evidence of aspiration of contrast material into the airway, the exploration did not proceed with administration of more liquid textures or higher volumes.
We designed an ad hoc survey, adapted from the Swallowing Quality-of-Life questionnaire (SWAL-QoL) for adults,17,18 and sent it electronically to the main caregivers of those patients who had completed 3 months of follow-up, after obtaining their consent. The survey was divided into two parts, the first referring to the situation prior to evaluation in the specialised clinic and the second after being assessed and receiving specific recommendations. The SWAL-QoL, which evaluates the self-perceived quality of life (QoL) of patients with dysphagia, has been developed and validated in several languages, including Spanish,18 and has been used to assess the impact of OPD in geriatric patients, those with head and neck cancer,19,20 Parkinson’s disease patients,21,22 stroke patients23 and adults with CP.24 In paediatrics, however, there is not yet a specific, validated tool for evaluating the QoL of patients with OPD.
The questionnaire on basic activities of daily living (BADLs) (Appendix B) was structured in 20 items, each with 5 possible answers rated on a Likert scale based on the frequency of the issue addressed in the question (1–5, from most unfavourable to most favourable), thus assessing how the individual completing the questionnaire perceived each of the dimensions. For the evaluation we grouped the items into 6 domains and subsequently converted the results into a percentage scale from 0 to 100 (the higher the percentage, the better the quality of life).
For the statistical analysis, we used the SPSS programme (SPSS Inc., Chicago, IL, USA). We determined whether the variables fit a normal distribution using the Shapiro-Wilk and Kolmogorov-Smirnov tests. We performed a descriptive study of the sample and variables calculating the mean and standard deviation or the median and interquartile range based on whether the data fit a normal distribution. We compared qualitative variables by means of the chi-square or the Fisher exact test, and quantitative variables using the Student t test for paired samples to compare means if the data were normally distributed or otherwise Wilcoxon signed-rank test. We used the Spearman rank correlation to analyse the association between ordinal variables, and the Kruskal–Wallis test to determine whether there were significant differences between the groups. We made comparisons with the Bonferroni correction for multiple comparisons. To evaluate the internal consistency of the survey we had formulated, we estimated the Cronbach alpha as a measure of the consistency of the items that made up the survey. We considered Cronbach alpha values higher than 0.6 acceptable. We considered P values of less than .05 statistically significant.
ResultsWe evaluated 103 patients, 56 (54.4%) of whom were male; 73.8% of them were Caucasian and 25.2% of Northwest African origin. The patients’ clinical features and source of referral are shown in Table 1; it should be noted that 85.4% of them had an underlying neurological pathology (CP, epileptic encephalopathy or neurodegenerative disorders, among others) and according to the Gross Motor Function Classification System (GMFCS) half the patients had a classification of III or less and the other half a GMFCS level of between IV and V. As regards external feeding devices, in the first evaluation 12 patients (11.6%) were wearing a nasogastric tube (NGT), one patient (1%) had a nasoduodenal tube (NDT) and 4 (4.2%) a percutaneous endoscopic gastrostomy (PEG), inserted with the percutaneous endoscopic approach in 3 and surgically in 1 (a patient that underwent Nissen fundoplication).
Clinical characteristics of patients evaluated at the OPD-C (n = 103).
| n | % | |
|---|---|---|
| Sex (M/F) | 56/47 | 54.4/45.6 |
| Age at initial evaluation in years, median (IQR) | 4.5 (1.8−9.4) | |
| Ethnicity (Caucasian/NW African) | 76/26 | 73.8/25.2 |
| Underlying disease | ||
| None | 4 | 3.9 |
| Cerebral palsy | 22 | 21.4 |
| Neurodevelopmental disorder | 8 | 7.8 |
| Neurodegenerative disorder | 16 | 15.5 |
| Epileptic encephalopathy | 16 | 15.5 |
| ABI | 5 | 4.9 |
| Craniofacial anomaly | 6 | 5.8 |
| Cardiorespiratory pathology | 3 | 2.9 |
| Prematurity | 2 | 1.9 |
| Genetic disease | 21 | 20.4 |
| Source of referral | ||
| Primary care | 3 | 2.9 |
| Specialist care | 89 | 86.4 |
| Gastroenterology | 41 | 46.6 |
| Paediatric neurology | 19 | 21.6 |
| Palliative care/Complex chronic disease unit | 9 | 10.2 |
| Respiratory medicine | 5 | 5.7 |
| Rehabilitation | 4 | 4.5 |
| Other | 10 | 11.36 |
| Other centre | 11 | 10.7 |
| Reason for referral | ||
| Cough on swallowing | 38 | 36.9 |
| Difficulty in expansion of oral diet | 21 | 20.4 |
| Possible chronic aspiration syndrome | 14 | 13.6 |
| Undernutrition | 12 | 11.7 |
| Other | 18 | 17.5 |
| GMFCS | ||
| I | 34 | 33 |
| II | 12 | 11.6 |
| III | 7 | 6.7 |
| IV | 11 | 10.7 |
| V | 39 | 37.9 |
| Recurrent respiratory infections (Yes/No) | 32 | 33 |
| Anthropometry at diagnosis (mean ± SD) | ||
| Weight z score | −1.5 ± 1.46 | |
| Height z score | −2.01 ± 2.06 | |
| BMI z score | −0.73 ± 1.49 | |
| z score differences after follow-up (mean ± SD/P value) | ||
| Weight z score difference | 0.11 ± 0.86 | 0.25 |
| Height z score difference | 0.14 ± 0.94 | 0.23 |
| BMI z score difference | 0.06 ± 1.06 | 0.63 |
| Duration of follow-up in months, median (IQR) | 6.54 (0.13−12.12) | |
| Use of external feeding device | ||
| At initial evaluation (Yes/No) | 16 | 16.7 |
| At final evaluation (Yes/No) | 24 | 26.1 |
ABI, acquired brain injury; BMI, body mass index; GMFCS, Gross Motor Functional Classification System; IQR, interquartile range; OPD-C, oropharyngeal dysphagia clinic; SD, standard deviation.
For the OPD evaluation, a clinical assessment was made by the medical specialist, since there was no speech therapist specialised in swallowing in the first year of operation of the specialised OPD clinic. All the patients underwent a complete anatomical and functional oral motor assessment and subsequent direct observation of oral intake. In cases that showed symptoms suggesting dysphagia, an instrumental test (VFSS) was prescribed.
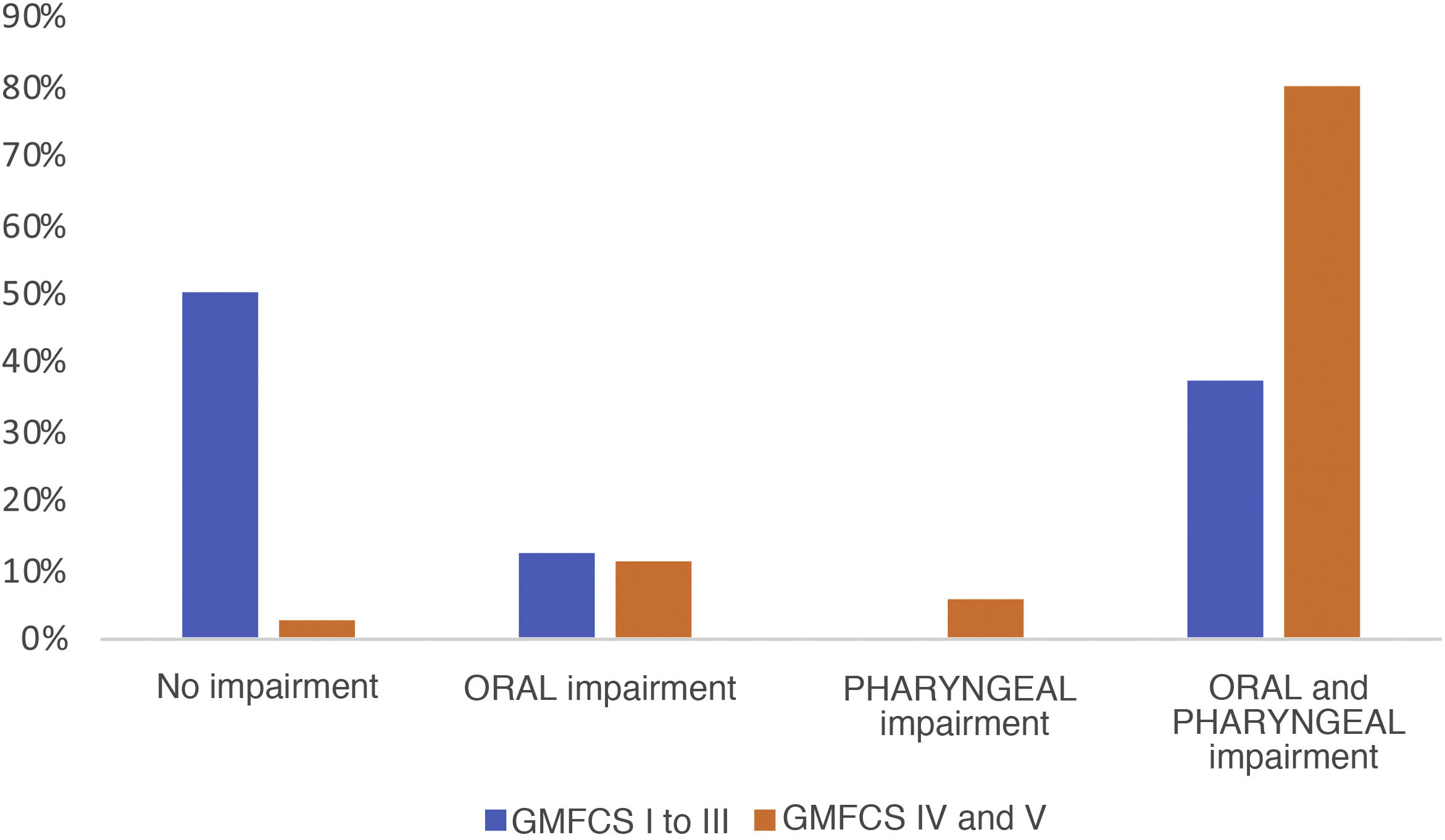
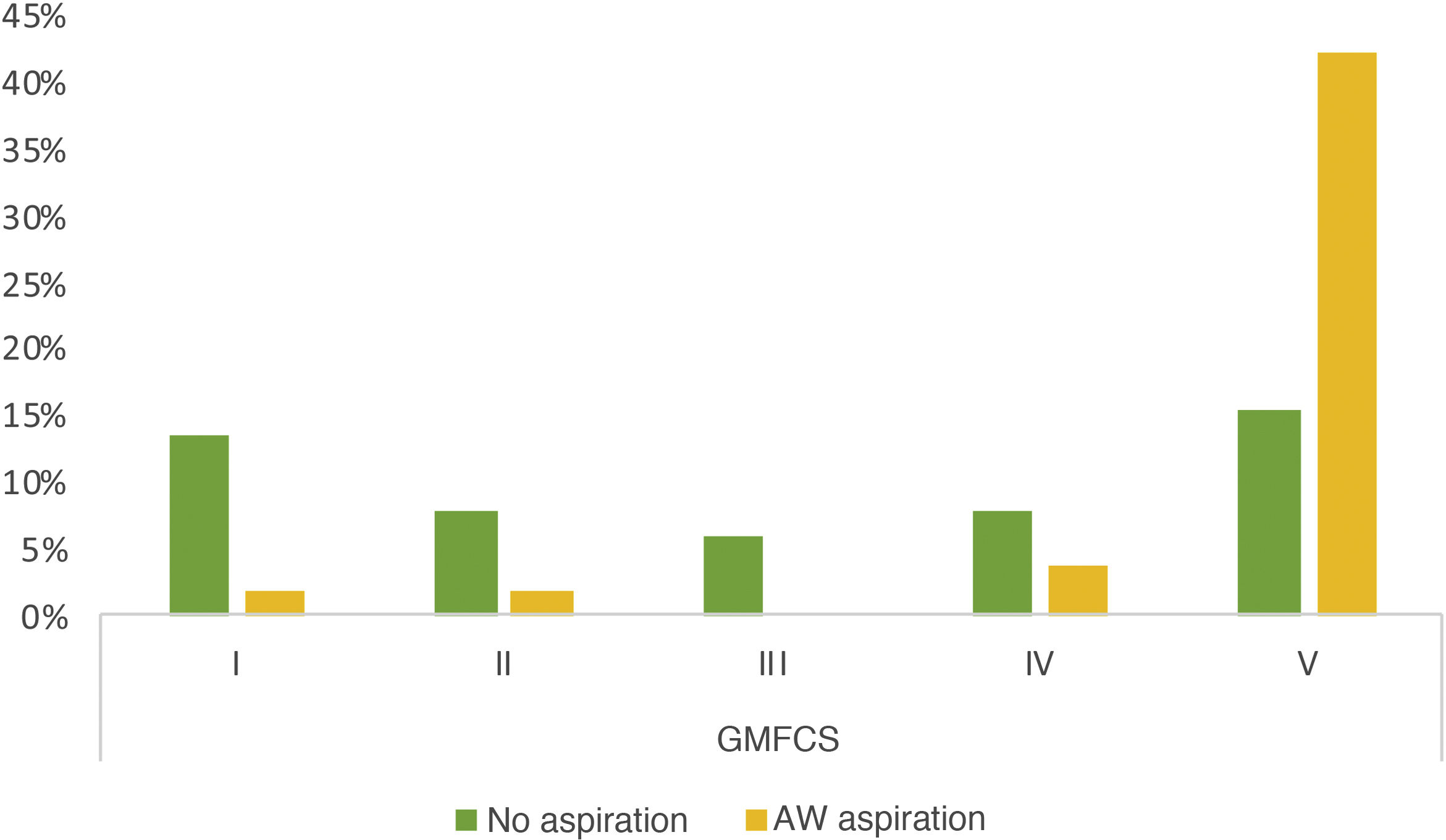
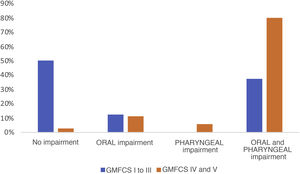
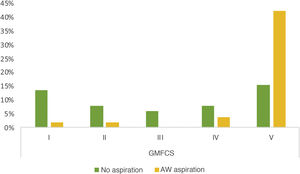
Of the 103 patients assessed, a VFSS was performed in 51 (49.5%), and 78.4% of these found pathological signs. Breaking them down by phases, 7 patients (13.7%) showed exclusive impairment of the oral phase, 2 patients (3.9%) of the pharyngeal phase and 34 (66.6%) abnormalities in both (Fig. 1). The proportion of patients with simultaneous impairment of the oral and pharyngeal phases (GMFCS groups IV and V) increased significantly with increasing degree of motor impairment (P = .021). We also observed a significant association between GMFCS and the presence of aspirations (P < .05). The prevalence of aspiration in children with greater motor impairment (GMFCS groups IV and V) was 52.2% higher than in children with a GMFCS level of III or less, a difference that was significant with a 95% confidence interval of 0.32 to 0.76. Aspiration was detected in 50% of the patients, and was silent in as many as 80.8% of cases (Fig. 2). There was a mean difference of 87.5% between the frequency of silent aspiration in patients with greater motor impairment (GMFCS IV and V) compared to those found in the group with lesser motor impairment (GMFCS ≤ III). No oxygen desaturations below 90% were detected in any patient during monitoring of the procedures. Looking in greater depth at the values obtained for the safety problems detected, using the Penetration Aspiration Scale (PAS), in 21.56% of the 51 patients who underwent a VFSS no entry of material into the airway was observed (PAS = 1), and 36.4% of these had a GMFCS level of I; penetrations into the airway, interpreted as a PAS score of between 2 and 5, were observed in 13 patients (25.5%), and aspiration (PAS from 6 to 8) in 27 patients (52.9%); in this group there was a significant association with the GMFCS, given that 22 of the 51 patients had GMFCS IV or V (ρ = 0.66; P < .05). We found a statistically significant difference between the various GMFCS groups with respect to the categorised PAS score (χ2(4) = 21.45; P < .01). In the post hoc analysis we found differences between GMFCS I and GMFCS V (P < .01).
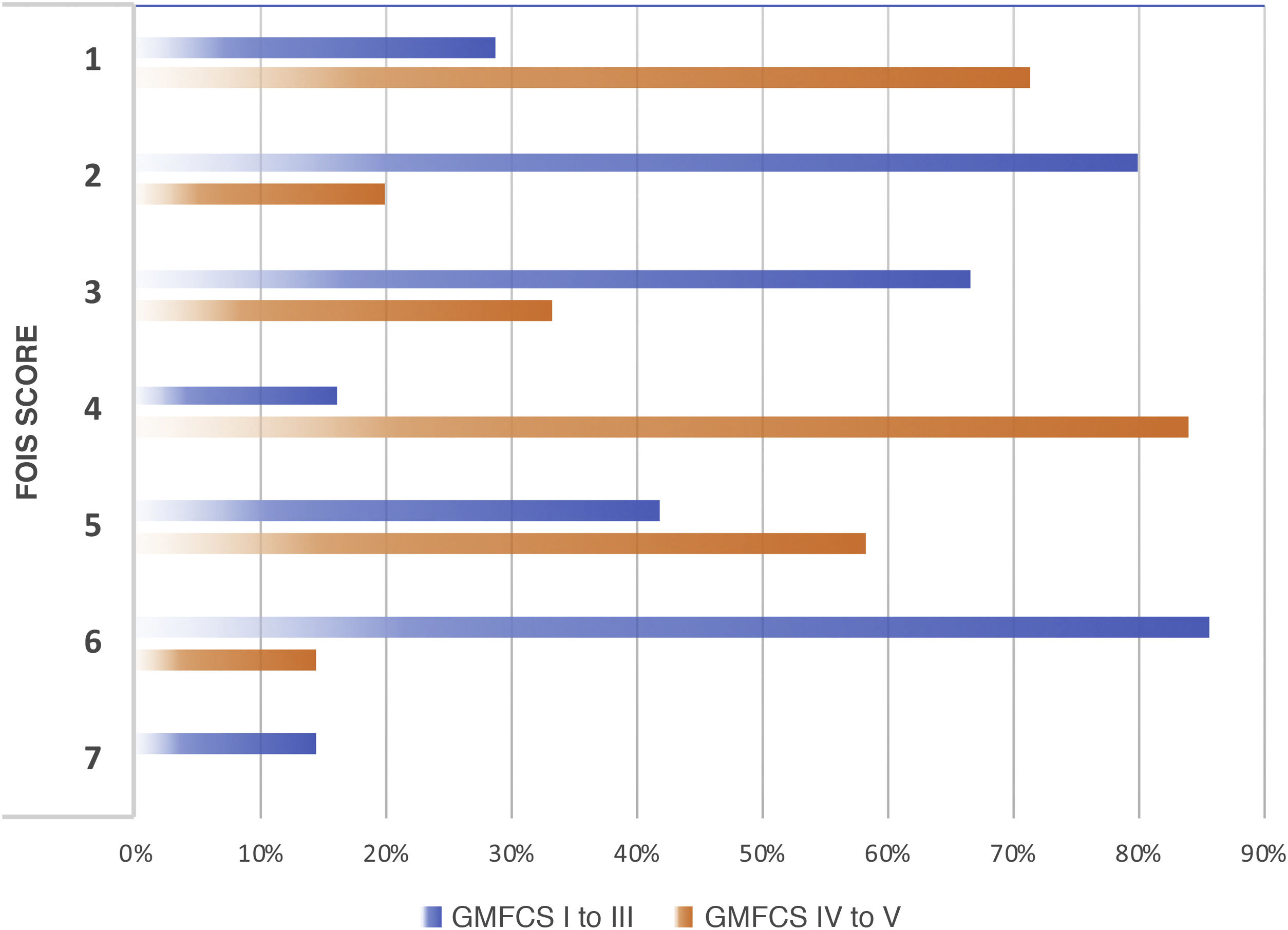
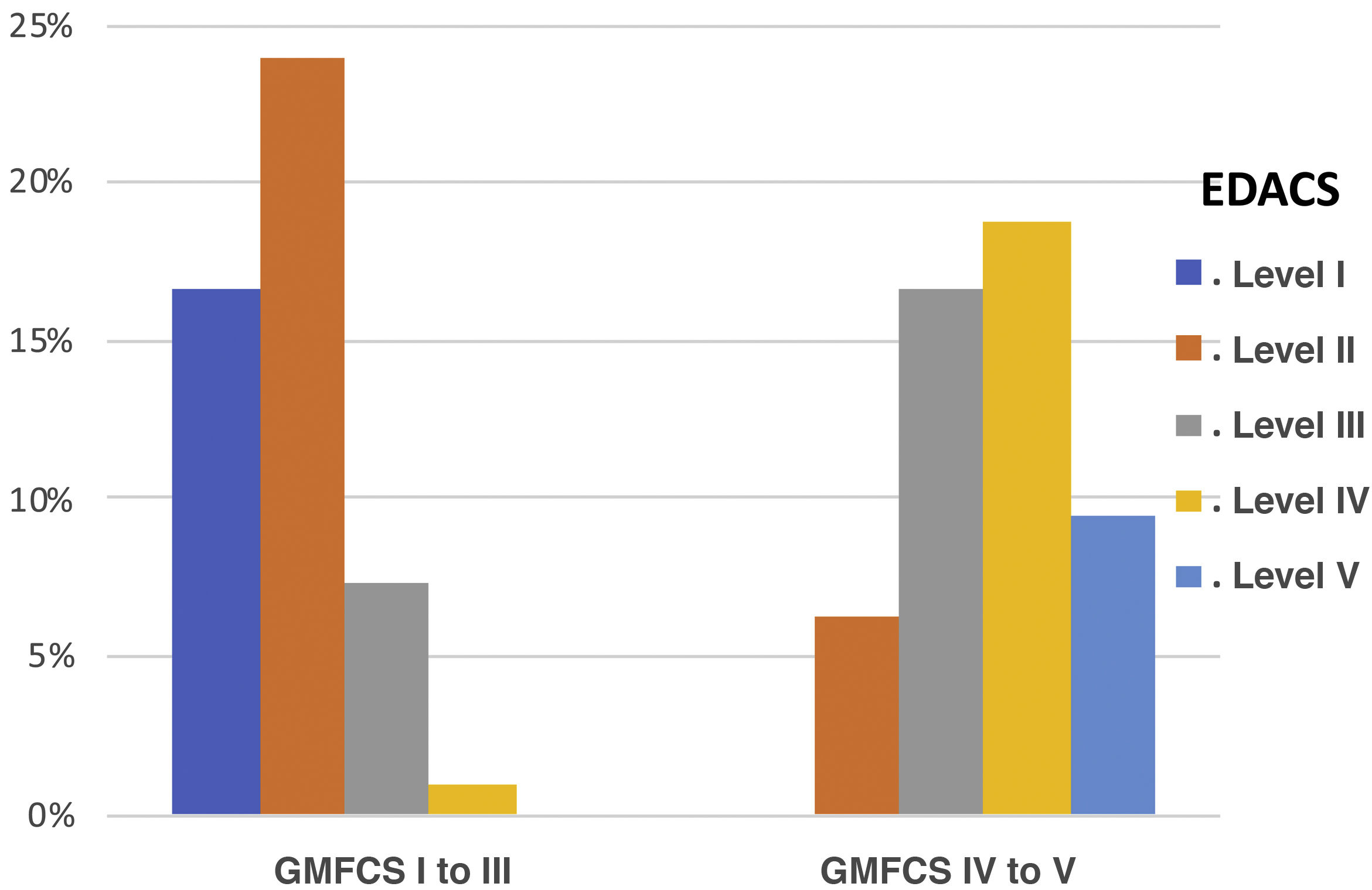
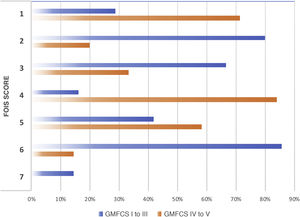
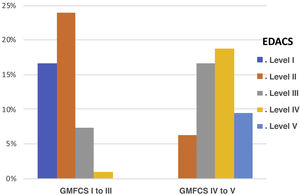
We observed a high and statistically significant correlation between the degree of motor impairment (GMFCS) and the severity of the dysphagia (evaluated with the EDACS scale) (ρ = 0.75; P < .05), and in this same connection we also obtained a statistically significant relationship between the GMFCS and the FOIS score (ρ = −0.44; P < .05) (Figs. 3 and 4). As for versions of the FOIS adapted for the paediatric population, no association was found in children aged under one year with the infant FOIS, and yet there was a moderate correlation with the paediatric version of the FOIS (ρ = −034; P < .05) and greater motor impairment. We found no significant differences between the median scores obtained with the FOIS severity scales in the initial evaluation (Me = 3) and the final follow-up assessment (Me = 2.5) on the patients’ last visit (z = −1.19; P > .05), either in the version adapted for the paediatric population (z = −1.23; P > .05) or in the infant version. Nor was there any change in the ability to eat and drink according to the EDACS scale (z = −0.58; P > .05).
Percentages of FOIS scores by GMFCS level. FOIS: Functional Oral Intake Scale. 1: nothing by mouth; 2: feeding-tube dependent (FTD) with minimal oral intake; 3: FTD with oral intake of food or liquids; 4: oral diet of a single consistency; 5: oral diet of multiple consistencies but requiring special preparation or compensations; 6: oral diet of multiple consistencies not requiring special preparation or compensations but with food restrictions; 7: oral diet without restrictions; GMFCS: Gross Motor Functional Classification System.
Percentages of EDACS scale scores by GMFCS group. EDACS: Eating and Drinking Ability Classification System. Level I: eats and drinks safely and efficiently; Level II: eats and drinks safely but with some limitations to efficiency; Level III: eats and drinks with limitations to efficiency and safety; Level IV: eats and drinks with significant limitations to safety; Level V: unable to eat or drink safely.
Of all the patients assessed, 55.4% worked with a speech therapist at an outpatient centre (early intervention programme or private practice), since there was no speech therapist specialised in swallowing in the first year of operation of the OPD clinic. After the patients had undergone a complete evaluation with thorough observation of food intake and a VFSS in the selected cases, the adaptation of textures and volumes in the diet was optimised for 51% of them on an individualised basis (according to the functional results obtained for safety and efficiency).
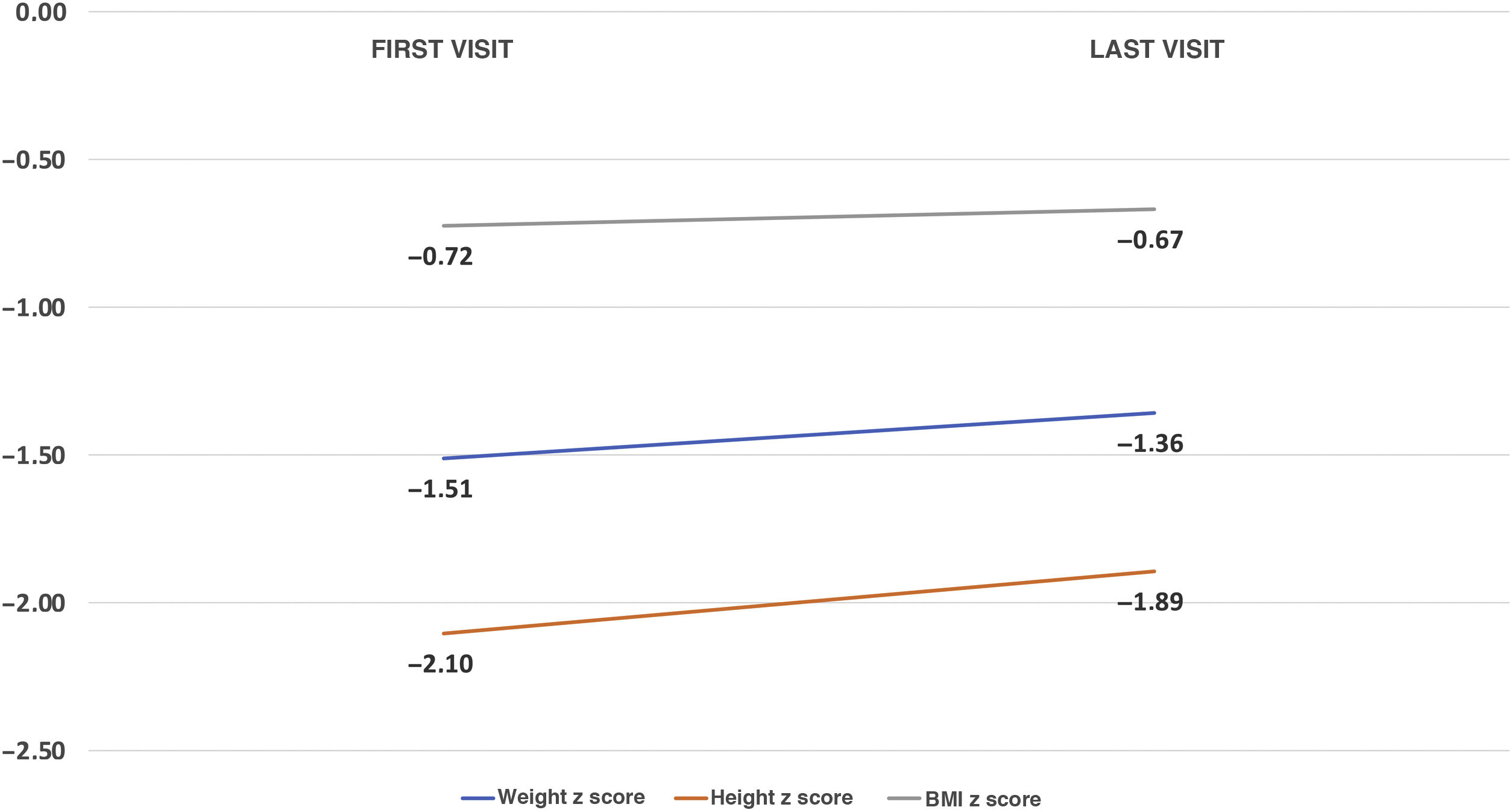
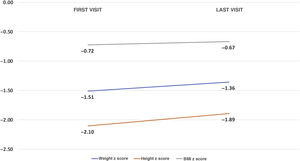
Median follow-up was 6.54 months (IQR, 0.13−12.12). An improvement was observed in all the anthropometric parameters: weight z score (−1.5 ± 1.46 to −1.36 ± 1.7; P = .21); height z score (−2,02 ± 2,06 to −1.85 ± 1.8; P = .15) and body mass index (BMI) z score (−0.73 ± 1.49 to −0.57 ± 1.62; P = .73) (Fig. 5). As for external feeding devices, placement of gastrostomy tubes was prescribed for 12 patients (12.6%) due to dysphagia that did not improve with compensatory techniques. The total percentage of patients with a gastrostomy tube in the final evaluation therefore amounted to 16.8%; 5 had been removed due to clinical improvement (preceded in all cases by a NGT). Seventy five percent of the gastrostomy tubes were placed by percutaneous endoscopy and the rest surgically (one patient received a Nissen fundoplication in the same operation and another underwent a gastrojejunostomy).
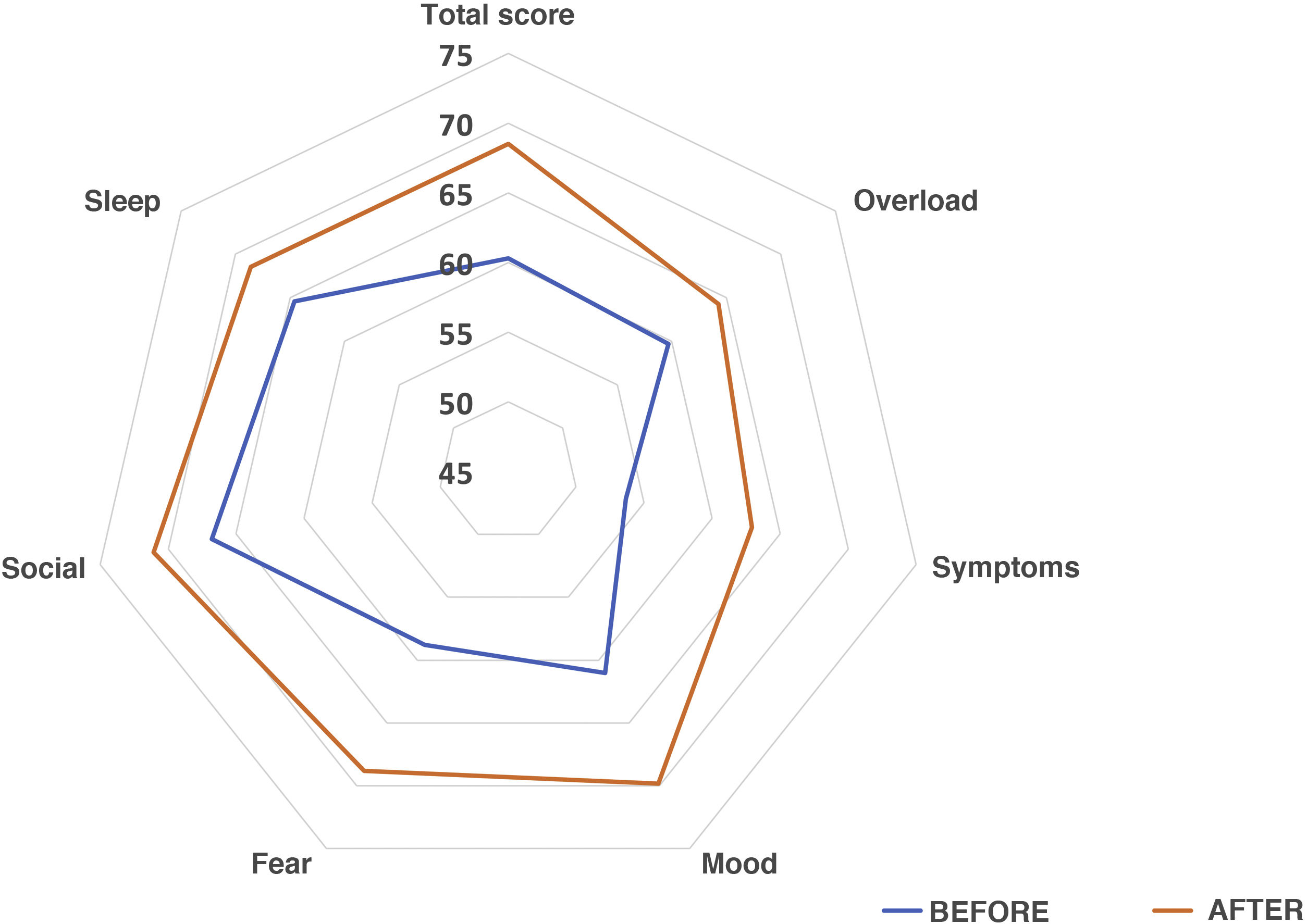
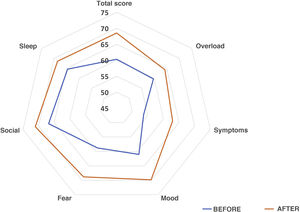
We sent questionnaires related to aspects of quality of life to the caregivers of 70 patients in the sample. Of this total, 74.3% answered both parts and 19% only the first (the latter were not taken into account in the peer analysis), and 88% of the caregivers found the questionnaire easy to answer. The results showed that 61% of those surveyed observed an overall improvement, 4.5% did not notice any changes, and 34.1% perceived a worsening. As for interference with BADLs, 46.2% of those surveyed responded that their children’s OPD problem affected them a lot or quite a lot, 26.9% little, 19.2% very little and 7.7% reported that had not found any notable interference. The overall increase in quality of life was statistically significant (Overall: 59 [IQR 51–71] vs 70 [IQR 51–83.5], P = .036]. By subdomains, an improvement was observed in all, except in sleep quality, which remained unchanged: Family overload: 60 (IQR 48–68) vs 64 (IQR 44–84), P = .31; Symptoms: 50 (IQR 30–70) vs 70 (IQR 50–80), P = .086; Mood: 56.6 (IQR 46.6–73.3) vs 71.6 (IQR 53.3–87.5), P = 0.04; Fear: 55 (IQR 30–80) vs 70 (IQR 57.5–90), P = .03; Social sphere: 70 (IQR 46,2–80) vs 80 (IQR 48.7–100), P = 0.39; Sleep quality: 80 (IQR 40–100) vs 80 (IQR 40–100), P = .65. Interference with BADLs remained unchanged: 60 (IQR 53.1–80) vs 60 (IQR 57.2–80), P = .40. Fig. 6 presents the percentage averages for each subdomain of the survey in a comparative form.
The reliability of the survey was very good; we obtained an internal consistency coefficient (Cronbach alpha) of .87 for the questionnaire as a whole; analysing by subdomains, the values lay in a range between α = .57 for the Symptoms subdomain and α = .79 for the Mood subdomain, which means that they complied with acceptable consistency levels. The question on sleep quality in isolation did not show a sufficient level of reliability (α < .5).
DiscussionA proper assessment of swallowing disorders in paediatric patients, using standardised instrumental techniques, and their subsequent follow-up at a specialised clinic are essential for a correct approach to and rehabilitation of these disorders, thereby reducing the morbidities that typically accompany these patients, such as undernutrition and possible respiratory complications.
The characteristics of the patients treated at paediatric dysphagia clinics and the numerous factors that interfere with the acquisition of self-feeding skills make the diagnosis and treatment of feeding problems particularly challenging and complex. The VFSS procedure is the gold standard for objective evaluation of deglutition,25–27 in anatomical and functional terms, as it enables us to view all the phases of swallowing (oral, pharyngeal and oesophageal), as well as to assess airway protection,2,28,29 although it is subject to not insignificant inter-practice variability due to the absence of properly standardised protocols. The standard exposure rate during a paediatric VFSS is normally 25–30 frames per second (fps), making it possible to identify brief penetration and/or aspiration events with high sensitivity.28,30 A reduction in radiation to 15 fps has already been evaluated in other studies,31 and though controversial, it is a suitable possibility with similar diagnostic yield to studies at 30 fps and would also support the ALARA (as low as reasonably achievable) radiation protection principle, reducing exposure to ionising radiation in children. With regard to the type of contrast agent, there is no uniformity; the most widely used are barium sulphate and iodine-based contrast media. Barium is the active principle most commonly employed in gastrointestinal and videofluoroscopic studies. Lower-density alternatives such as the iso-osmolar water-soluble contrast agent iodixanol, as described in our series, have previously been reported in the literature,25,32 and have proved to be well tolerated and safe.
In a study conducted in a population similar to ours32 comprising 61 children with OPD, abnormalities were observed in the oral phase after a VFSS was performed in 50% of the patients, in the pharyngeal phase in 67%, and only 3% of the patients evaluated had abnormalities in the oesophageal phase. These results are very similar to those obtained in our sample with respect to abnormalities in the oral and pharyngeal phases, which are those described in our study. As many as 65.4% of the patients showed abnormalities in both phases (oral and pharyngeal) simultaneously. As in our study, the presence of aspiration was more common in the population with greater neurological impairment; similar findings have been described in other series.32,33 One of the abnormalities frequently detected in the VFSS is penetration of food into the pre-epiglottic space, which may be a marker of subsequent aspiration of that food into the airway. In a study34 conducted in 125 children with suspected OPD, data were analysed after performing a VFSS and it was observed that as many as 81% of the patients showed laryngeal penetration, 31% being deep penetrations, ultimately with aspiration of content into the airway in 85% of them. The results obtained in our study enable us to support these data by stating that such aspiration events are more common in patients with greater motor impairment (according to the GMFCS scale), and highlighting the fact that a high proportion of these aspiration events are silent, with no activation of the cough reflex or oxygen desaturation, and therefore a proper diagnosis is essential to avoid respiratory complications.
In patients with neurological deficits, such as children with CP, it is common to find evidence of malnutrition, multifactorial in origin but in which the presence of OPD is a constant in groups with greater motor impairment. In a recent series with 260 children with CP it was shown that 38% were undernourished and that this prevalence increased with the score on the GMFCS scale;35 similar data have been collected in other previous studies.36 As in our series, it was shown that after proper evaluation and treatment of their swallowing disorder there was a significant improvement in the parameters,37 although further studies will be required to ascertain other factors that may be exerting an influence.
A recent study38 analysed the difficulties perceived by caregivers of patients with moderate to severe CP in pursuing BADLs, concluding, in line with previous studies,39 that the prevalence of comorbidities such as OPD and severity of neurological impairment of these patients directly undermines the caregivers’ quality of life. Moreover, OPD and nutritional deficit usually go together, and this is undoubtedly another factor that contributes to the difficulty of care.40 The caregiver’s quality of life is becoming an essential component of the cost-effectiveness analysis of interventions in children with CP.39 In the same vein, our data seem to support the idea that optimisation of proper diagnosis of OPD and early initiation of the most suitable specific dietary recommendations and adaptations in patients may help both to improve the nutritional situation and to enhance the perception of improvement in quality of life for caregivers.
Setting up a specialised OPD clinic at our hospital has facilitated collaboration with radiologists, rehabilitation specialists, a speech therapist and a dietician-nutritionist, shaping the creation of a multidisciplinary unit for addressing paediatric patients with OPD.
As regards limitations, the main weakness of our study is its retrospective nature. The first data obtained from the pilot survey on caregivers’ quality of life shows a good internal reliability level, despite the low sample size and low number of items in some subdomains. More studies will be needed to be able to validate the survey as a more robust questionnaire that measures the self-perception of the quality of life of caregivers of paediatric patients with OPD.
In conclusion, correct and objective evaluation and treatment of paediatric OPD is essential to be able to provide families guidance on how to feed patients and to develop individualised rehabilitation programmes. Ensuring the safest and most effective feeding route for each patient and seeking ways of compensating, when possible, for the swallowing abnormalities observed in the evaluation are crucial. It is also essential to continue investigating new reliable assessment techniques that enable us to be more specific in describing abnormalities in the phases of swallowing, as well as incorporating staff who specialise in oral motor assessment and rehabilitation to be able to address the treatment of these patients with an interdisciplinary approach. Furthermore, implementing action protocols in every paediatric centre that deals with patients with swallowing disorders is a vital task that would improve the quality of life of patients and their families
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ortiz Pérez P, Valero Arredondo I, Torcuato Rubio E, Rosa López A, García-Herrera Taillifer P, Navas-López VM. Caracterización clínico-patológica de niños con disfagia, impacto familiar y calidad de vida de sus cuidadores. An Pediatr. 2022;96:431–440.