Vaccinations are one of the main public health tools for the control of vaccine-preventable diseases. If a child is labelled to have had an allergic reaction to a vaccine, the next immunizations will probably be suspended in that child, with the risks involved in this decision. The rate of severe allergic reactions is very low, ranging between 0.5 and 1/100,000 doses. The causes of allergic reactions to vaccines, more than the vaccine itself, are often due to residual protein components in the manufacturing process, such as gelatin or egg, and rarely to yeast or latex. Most of vaccine reactions are mild, localised at the site of injection, but in some circumstances, severe anaphylactic reactions can occur. If an immediate-type allergic reaction is suspected when vaccinating, or a child allergic to some of the vaccine components has to be vaccinated, a correct diagnosis of the possible allergy has to be made. The usual components of each vaccine should be known, in order to determine if vaccination can be performed safely on the child.
Las vacunaciones constituyen una de las principales herramientas de salud pública para el control de las enfermedades inmunoprevenibles. Si un niño es etiquetado de haber presentado una reacción alérgica a una vacuna es probable que se suspendan las siguientes inmunizaciones, con los riesgos que ello conlleva. La tasa de reacciones alérgicas graves es muy baja, oscilando entre 0,5 y 1/100.000 dosis. Las proteínas causantes de las reacciones alérgicas, más que los propios antígenos vacunales, son frecuentemente componentes residuales del proceso de fabricación, como son la gelatina y el huevo, y más raramente las levaduras o el látex. La mayoría de las reacciones son leves y localizadas en el lugar de la inyección, aunque en algunos casos pueden producirse reacciones anafilácticas graves. Si se sospecha que se ha producido una reacción alérgica inmediata a la vacuna, o si debemos vacunar a un niño con alergia a alguno de sus componentes, se deberá realizar un correcto diagnóstico de la posible alergia y conocer los componentes habituales de cada vacuna con el fin de determinar si la vacunación puede continuarse de forma segura.
The aim of vaccination programmes is to protect the vaccinated child and prevent the recipient from having the disease against which he or she was vaccinated. The goal is to vaccinate the greatest possible number of susceptible individuals to produce a collective protective environment for the entire population.
If a child is labelled as having had an allergic reaction to a vaccine (ARV), subsequent vaccinations will probably be suspended and that child will join the pool of individuals susceptible to the diseases against which he or she has stopped being vaccinated. Therefore, diagnosing the ARV accurately and confirming whether there is a direct relationship between the allergic reaction and the administration of the vaccine are of the essence.
The approach to a child with suspected ARV should start by determining whether the signs and symptoms presented by the child were directly related to the administration of the vaccine, managing the allergic reaction, and then assessing whether the adverse event was a reaction against the vaccine antigen itself or to any of the vaccine components, as this will determine the administration of future doses of the vaccine in question or similar vaccines.1
There is a wide variety of ARV. They are usually mild, local reactions, and exceptionally severe anaphylactic reactions that may even be fatal.2
Vaccine composition: antigens, preservatives and adjuvantsVaccines do not only contain the antigen responsible for stimulating the immune response in the vaccinated individual, but may also contain additional constituents (Table 1).
Epidemiological data. Incidence of allergic reactions after vaccinationAllergic reactions to vaccines are rare and usually have no causal relationship with immunisation. Estimates of true immediate hypersensibility reactions to vaccines range from 1 in 50000 doses for the DTP vaccine to 0.5–1 reaction per million doses for other vaccines.1 The Brighton Anaphylaxis Working Group estimates that the number of true severe anaphylactic reactions to vaccines ranges between 0.5 and 1 in 100000 doses.3 The number of reported deaths due to anaphylaxis secondary to vaccination is approximately 1 in 50 million doses.4
There are few published data for Spain, with reported incidences ranging between 0.59% and 1.27% of reactions suspected to be associated with vaccination in the first visits to a paediatric allergy unit.2
Among the allergic reactions attributed to residual proteins, the most common are those related to egg in vaccines grown in both chicken embryo fibroblasts and fertilised chicken eggs.
It is convenient to complete a questionnaire before vaccination5 asking the parents of the child that is to be vaccinated about the presence of certain circumstances that may lead to consider the vaccination to be contraindicated either temporarily or permanently, or to take specific precautions (Table 2).
Pre-vaccination questionnaire.
| Delay vaccination: | |
|---|---|
| Has the patient been ill in the past few days? | |
| Acute moderate or severe disease | Until patient recovery or stabilisation |
| Fever>38.5°C | |
| Acute allergy or asthma episodes | |
| Cancer | |
| Acute decompensated heart failure | |
| Acute nephropathies | |
| Measles | 2 months after onset of rash |
| Active tuberculosis | 2 months after initiation of treatment |
| Progressive or unstable neurological disorder, or one predisposing to seizures, such as acute encephalitis, tuberous sclerosis or uncontrolled epilepsy | Delay pertussis vaccination until neurological condition has stabilised |
| Contraindicated: | |
|---|---|
| Primary immunodeficiency, solid or blood tumours, long-term immunosuppression | MMR, varicella, rotavirus |
| HIV infection with severe immunodeficiency (CD4+ T-lymphocyte percentage <15% for age) | MMR, varicella, rotavirus |
| History of intussusception or congenital gastrointestinal malformation | Rotavirus |
| Precaution: | |
|---|---|
| Disease or treatment leading to coagulation abnormalities or thrombocytopaenia | For parenteral vaccines, use the subcutaneous route when allowed by the summary of product characteristics. If administration must be intramuscular, use 25G or 23G needles only, apply pressure to the injection site for 2min, do not massage injection site and watch for subsequent development of haematoma |
| Delay vaccination (from the time treatment ends) | |
|---|---|
| Is the patient currently taking any medications or receiving any kind of treatment? | |
| Blood productsGamma globulinsMore time for very high doses of gamma globulin (consult) | 3–6 months for MMR and varicella (depending on the preparation) |
| High-dose systemic corticosteroids (≥2mg/kg or 20mg a day of prednisone or equivalent) for more than 2weeks or ≥1mg/kg for more than 1monthNo need to delay vaccination if corticosteroid therapy is short-term, nonsystemic, low-dose, given every other day or is a replacement therapy | 1month for MMR and varicella |
| Acyclovir, famciclovir, valacyclovir | One week for varicella |
| If female, is the patient pregnant or suspects that she is? | |
| If pregnant | MMR and varicella are contraindicated |
| If in the first trimester of gestation | Avoid all vaccines, except for the influenza vaccine (evaluate specific risk) |
| Has the patient received any other vaccines previously? | |
| If patient has received any live attenuated vaccine shots (MMR, varicella, yellow fever) | Wait a minimum of 4 weeks before administering another live attenuated vaccine (MMR, varicella, yellow fever) |
| Does patient have a history of severe reaction to vaccines? | |
| Severe allergic reaction (anaphylaxis) following a previous dose of the vaccine or to a vaccine component | The vaccine in question is contraindicated |
| Encephalopathy within 7 days from administration of the DTP/DTaP vaccine in the absence of any other identifiable cause | Pertussis vaccine contraindicated |
| One of the following after DTP/DTaP vaccine:- Fever>40.5°C, hypotonic-hyporesponsive episode, inconsolable crying lasting ≥3h, within 48h of vaccination- Seizures within 3 days of vaccination | Exercise caution with pertussis vaccine |
| Guillain-Barré syndrome in the 6 weeks following vaccination | Exercise caution with vaccine in question |
| Arthus reaction after a dose of vaccine containing tetanus toxoid | Delay any other doses of tetanus vaccine for at least 10 years |
| Contraindicated: | |
|---|---|
| Is the patient allergic to any of the vaccine components? | |
| Only in case of anaphylaxis (immediate and potentially serious reaction) | |
| Allergy to neomycin | Hepatitis A (Havrix®), hepatitis A+B, hexavalent vaccines, influenza (Chiroflu®, Chiromas®, Dotaricin®, Inflexal V®, Intanza®, Mutagrip®, Vaxigrip®), pentavalent vaccines, injectable polio, rabies, MMR and varicella |
| Allergy to streptomycin | Hexavalent (Hexyon®), pentavalent (Pentavac®) and injectable polio |
| Allergy to polymyxin B | Influenza (Inflexal V®), hepatitis A (Epaxal®), hexavalent, pentavalent and injectable polio |
| Allergy to gentamicin | Influenza (Fluarix®, Fluenz®, Influvac®) |
| Allergy to kanamycin | Influenza (Chiroflu®, Chiromax®, Dotaricin®) |
| Allergy to gelatin | Typhoid fever (Vivotif®), MMR (MMRVaxpro®) and varicella (Varivax®) |
| Allergy to baker's yeast | Hepatitis A+B, hepatitis B, hexavalent vaccine and HPV4 (Gardasil®) |
| Allergy to egg proteins | Central European encephalitis, yellow fever, influenza, hepatitis A (Epaxal®) and rabies (Rabipur®). Egg allergy is not a contraindication to the MMR vaccine |
HPV4, tetravalent human papillomavirus; MMR, measles mumps rubella.
Source: Adapted from Fernández Cuesta et al.5
An allergic reaction is defined as an adverse response produced by an immune mechanism. It can include a wide range of symptoms affecting the skin and the respiratory and cardiovascular systems. Anaphylaxis can be confirmed by determination of serum tryptase levels.
Allergy is much less frequent than other types of adverse reactions. In many instances, the ARV is not confirmed and the patient may continue the course of vaccination with the same preparation.6
Reactions are classified, depending on their reach, into local or systemic and, depending on the time elapsed since the administration of the vaccine and the development of symptoms, into immediate or delayed. This criterion helps to distinguish IgE-mediated reactions from those that do not involve IgE.1
Immediate reactionsThey start within an hour from vaccination (from a few minutes to 4h). They may include a wide range of symptoms affecting the skin, the respiratory system and the cardiovascular system.
Delayed reactionsThey start hours or days after vaccination and there is a very low probability that they are mediated by IgE. They are not usually caused by an immune mechanism and should not be diagnosed as allergies to vaccines. They are self-limiting processes that do not contraindicate future doses of the same vaccine.
Differential diagnosis of allergic reactions during and after vaccine administrationA previous diagnosis of ARV is important for two reasons: firstly, individuals that experienced an IgE-mediated reaction, and especially anaphylaxis, could experience new severe reactions after vaccination; and secondly, overdiagnosis of ARV could increase the number of children that do not complete vaccination, which carries an individual and collective loss of protection against vaccine-preventable diseases.
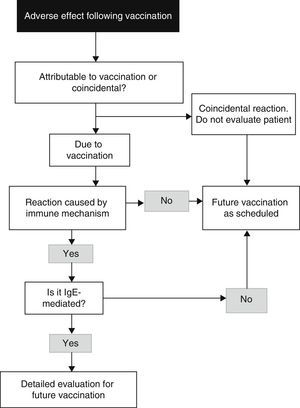
The algorithm for the approach to diagnosis can be seen in Fig. 1.7
Algorithm for the identification of an allergy reaction to vaccines (modified from Kelso et al.7).
Delayed reactions are nonspecific, and serum IgE and skin tests are usually not helpful; although these reactions cause discomfort, they are not life-threatening.8
Vaccine components that may cause allergic reactionsThe components of vaccines marketed in Spain that may be involved in AVR can be found in the online manual of vaccines of the AEP (http://vacunasaep.org/documentos/manual/anx-ii9). None of the vaccines currently marketed in Spain contains thiomersal.
Vaccination of children with a history of possible allergic reaction after administration of a vaccine. Action guidelineAn accurate diagnosis of VAR is based in the medical history and in vitro and in vivo allergy testing.10
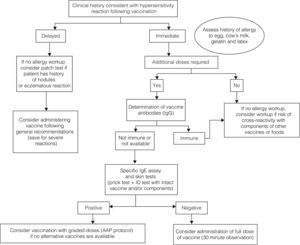
The diagnostic algorithm is presented in Fig. 2.
The medical records will help determine whether the hypersensibility reaction experienced by the child was immediate or delayed.
- •
If an IgE-mediated immediate reaction is suspected, allergy testing should be done, especially if additional doses of the vaccine are required and to avoid the risk of cross-reactivity with components of other vaccines or foods.10
- •
If a non-immediate reaction is suspected, the vaccine can be given in the usual manner in most cases.8
The determination of IgG antibodies against the immunising agent can be useful if the patient has received fewer than the recommended number of doses in order to assess the level of immunity. If a patient has protective levels of antibody, subsequent doses could be withheld, although the duration of immunity might be shorter.7 These levels have been determined for some vaccines (Table 3).
Protection levels of antibodies to vaccine (IgG).
| Vaccine | Protective level of IgG antibody |
|---|---|
| Diphtheria | ≥0.1IU/mL |
| Haemophilus influenzae b | ≥0.15μcg/mL |
| Hepatitis A | ≥10mIU/mL |
| Hepatitis B surface antibody | ≥10mIU/mL |
| Measles | ≥120 PRN titre |
| Polio types 1, 2 and 3 | ≥1:8 neutralising antibody titre |
| Rabies | ≥0.5IU VNA/mL |
| Rubella | ≥10IU/mL |
| Tetanus | ≥0.1IU/mL |
| Yellow fever | ≥0.7IU/mL |
PRN, plaque reduction neutralisation; VNA, virus-neutralising antibodies.
Source: Kelso et al.13
If additional doses are required or the level of protective antibodies cannot be determined, allergy testing should be done for the intact vaccine or for specific components if an allergy to those components is suspected.
- •
If test results are negative, administration of a full-strength dose of vaccine will be considered, keeping the patient under observation for 30min.
- •
If test results are positive, administration in graded doses will be considered if a vaccine preparation without the component to which the patient is allergic is not available.10
- •
Detailed history of the adverse reaction: patient age, date of reaction, brand of the administered vaccine, lot number, latent period, symptoms, urgent medical care, treatment received, duration of reaction.
- •
Vaccine composition.
- •
Simultaneous exposure to other allergens: medications, foods, latex, etc.
- •
Previous or subsequence tolerance to other vaccines.
- •
Tolerance to vaccine components.
Skin tests (STs) start with a full-strength skin prick, and if the result is negative an intradermal (ID) test with a 1:100 dilution in normal saline is performed.11
Allergy workup of adverse reactions to vaccines.
| Skin allergy tests: |
| Intraepidermal puncture or “skin prick” testing |
| Intradermal testing (ID) |
| Epicutaneous or patch testing (24). |
| Specific IgE antibody serology assays with the ImmunoCAP (RAST) method, considered positive for values above 0.35kU/L |
In cases of severe anaphylactic reaction, testing should start with a 1:10 concentration skin prick and a 1:1000 dilution for the ID test.
The sensitivity and specificity of vaccine STs for confirming or ruling out allergy to a vaccine or its components have not been established, but if the ST results are negative it is very improbable that the patient has IgE antibodies against the vaccine or its components.
If more than one year has elapsed since the IgE-mediated reaction, there may be low levels of circulating IgE and the ST results may be negative, so administration of the vaccine may not cause a reaction, although if the patient is sensitised it can trigger a booster response and a reaction may occur when the vaccine is administered after a long period of time.12
Investigation of sensitivity to vaccine componentsIf the result of the ST with the intact vaccine is positive, the patient will be tested for sensitivity to vaccine components to try to prevent reactions to other vaccines that have the same components.
The available tests for vaccine components are:
- a.
Tetanus toxoid: determination of specific IgE serum levels (sensitive, but with low specificity).
- b.
Egg: ST and determination of specific IgE serum levels for egg white and ovalbumin.
- c.
Cow's milk: ST with extracted cow's milk proteins: bovine alpha-lactalbumin, beta-lactoglobulin, casein and serum albumin; specific IgE antibody assay for the same proteins.
- d.
Gelatin: nonstandardised ST using commercial gelatin powder (5g of gelatin dissolved in 5mL of normal saline)13; specific IgE antibody assays.
- e.
Latex: latex ST and specific IgE antibody assay.
Sensitivity testing for vaccine components.
Patients with a history of eczematous reaction to a vaccine containing phenoxyethanol or formaldehyde:
a. Patch test with standardised concentration
Patients that have had an eczematous reaction or persistent injection-site nodules with an aluminium-containing vaccine:13a. Patch test with:
- i.
Metallic aluminium using an empty Finn Chamber.
- ii.
Aluminium chloride hexahydrate 2% in glycerine placed in a plastic chamber.
If vaccine or vaccine component allergy test results are negative, the vaccine can be administered in a single dose keeping the patient under observation for a minimum of 30min afterward. If there is a history of severe reaction, the vaccine will be administered in two doses: a first dose with a 1:10 dilution of vaccine followed 30min later by the rest of the full dose, keeping the patient under observation for at least another 30min. There are no published cases of patients with negative vaccine ST results that had a severe anaphylactic reaction following revaccination.
If the ST or specific IgE assay results are positive in a patient with a history of IgE-mediated reaction to one of the components of the vaccine, a preparation that does not contain that component will be used whenever possible. If it is not available and the administration of the suspect vaccine or another vaccine containing the suspect component is necessary, the vaccine will be administered in graded doses in a hospital setting, following the protocol shown in Table 5.
Dose-escalating administration of the vaccine.
| 1st dose: | 0.05mL of 1:10 dilution in normal saline |
| 2nd dose: | 0.05mL full-strength |
| 3rd dose | 0.10mL full-strength |
| 4th dose | 0.15mL full-strength |
| 5th dose | 0.20mL full-strength |
| 6th dose | 0.5mL dose (for vaccines requiring a 1mL volume) |
Recommendations of the American Academy of Paediatrics.16
If the allergy workup is inconclusive and the reaction occurred after the simultaneous administration of several vaccines, revaccination must be performed administering each of the vaccines on a different day.
Revaccination of patients with delayed allergic reactions14,15Revaccination must be considered on the basis of its necessity and the nature and severity of previous adverse reactions. If the vaccine is administered, the patient must stay under observation for 30min.
If several vaccines had been administered simultaneously, revaccination must be carried out by administering each of the vaccines on a different day, starting with the vaccine suspected to be the least risky.
It has been demonstrated that STs are not useful in predicting the development of delayed reactions in subsequent administrations of the vaccine.
In patients that had persistent injection-site nodules after administration of a vaccine containing aluminium salts and with positive patch-test results, vaccines that do not contain these salts should be used whenever possible. If the child is sensitive to aluminium salts and needs a dose of a vaccine that contains them, it is recommended that the vaccine be administered with a deep intramuscular injection to minimise the local reaction.
Vaccination of children with suspected or confirmed allergies to vaccine components. Action guidelineWhen vaccination is considered in a child, he or she may be allergic to one or more of the residual proteins from the manufacturing process or to any other product used in its preparation. There is no evidence that children with atopy are at greater risk of having allergic reactions following vaccination, and they should receive all the recommended vaccinations.16,17
Children with allergy to egg proteinsEgg allergy is the leading food allergy in children,18 with an estimated prevalence of 2.5% in the first 2 years of life.19 The measles-mumps-rubella (MMR) vaccine is growth in chicken embryo fibroblasts, while the influenza vaccine and the yellow fever vaccine are cultured in fertilised eggs that may contain greater amounts of egg proteins. Other vaccines that contain egg proteins are the hepatitis A vaccine Epaxal® and the vaccine against Central European encephalitis. One of the vaccines against rabies marketed in Spain is not cultured in chick embryo cells, but in human diploid cells.
Measles mumps rubella vaccineIt does not contain egg proteins capable of triggering an allergic reaction, and therefore all children with egg allergies, including those who have anaphylactic reactions, should be given this vaccine in the setting where they are routinely vaccinated.20 Children that have had a reaction to a previous dose of MMR vaccine should be assessed by an allergist or paediatric allergist. These reactions are triggered by other vaccine components.
Influenza vaccineAdministration of the influenza vaccine is recommended in children older than 6 months in certain at-risk groups.21 If egg has yet to be introduced in the child's diet and it is suspected that the child may be allergic, the child should be evaluated by a paediatric allergist prior to vaccination.
The influenza vaccine may contain traces of ovalbumin,22 and since it does not undergo heat treatment during manufacturing, heat-labile egg proteins remain intact and may trigger reactions even in children that tolerate cooked eggs.
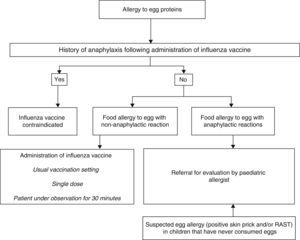
In cases of anaphylaxis following vaccination against influenza, no additional doses will be administered (Fig. 3).
In cases of egg allergy without severe anaphylaxis, it is safe to administer an influenza vaccine with less than 0.6–1μg of ovalbumin per dose,23 so the vaccine can be administered in the usual setting24,25 in a single dose, and prior skin testing is unnecessary.26 It has been demonstrated that immunisation against influenza with vaccines with a low content of ovalbumin without dividing the dose is safe in patients with egg allergy with severe anaphylactic reactions, and vaccines with a high ovalbumin content have even been used without complications in children with anaphylactic reactions to egg in the diet.27–29
Yellow fever vaccineIt is cultured in chicken embryos, so it may contain significant amounts of egg proteins.30 If administration of the vaccine is necessary, children with egg allergies should be evaluated by an allergist or paediatric allergist. If test results are negative, the patient can be vaccinated in the usual manner, and if they are positive and vaccination is absolutely necessary, the vaccine must be administered in graded doses in a hospital setting.31,32
Children with cow's milk allergyThe presence of milk derivatives in vaccines is very rare. The summaries of product characteristics of the vaccines marketed in Spain do not mention the potential presence of cow's milk proteins, although inadvertent contamination with proteins from the culture medium may occur. Lactose may be added as an excipient to some vaccines, but these are still free of cow's milk proteins. From a practical standpoint, it is safe to vaccinate a child that has a cow's milk allergy.
Children with allergies to antibiotics, gelatin, fungi, yeasts and aluminiumNeomycin and other antibioticsAminoglycosides (gentamicin, kanamycin), polymyxin, chlortetracycline and neomycin are added to vaccines to avoid bacterial contamination during the manufacturing process.
Neomycin can cause systemic allergic reactions that contraindicate the administration of vaccines containing it.33 It can also cause local reactions, such as contact dermatitis, that require much greater amounts of neomycin than those usually contained in vaccines, and that do not contraindicate vaccination.34
There have been no reports of reactions to any of the other antibiotics triggered by vaccination.35,36
GelatinGelatin is an animal protein obtained from the connective tissue in cows and pigs. It is used as a stabiliser in live-attenuated virus vaccines.7 If the patient is allergic to gelatin, a vaccine skin prick test should be performed prior to vaccination. If the result is positive and no other preparation is available that does not contain gelatin, the vaccine will be administered in graded doses, and if the result is negative the vaccine will be given in the usual manner.13
Fungi and yeastsThe hepatitis B vaccine and one of the human papillomavirus vaccines are manufactured by harvesting the antigens from cell cultures of recombinant Saccharomyces cerevisiae strains. The amount of yeast contained in a vaccine can be of up to 5mgpermL in the hepatitis B vaccine and is smaller in the human papillomavirus vaccine.7 If there is suspicion of a yeast allergy in the patient, a skin prick test and a specific IgE assay need to be performed. If the results are negative, vaccination can be done in the usual manner. If the results are positive and vaccination is absolutely necessary, the vaccine should be administered in graded doses.16
AluminiumAluminium is used as an adjuvant to enhance the immune response.37 The reaction usually triggered by aluminium consists of painful itching nodules at the site of injection that do not contraindicate vaccination.38 There are very few references to generalised eczema in the literature,39 so there is no scientific evidence to justify not recommending vaccination in children with a sensitivity to aluminium diagnosed by patch testing.40
Children with a latex allergyChildren with a confirmed allergy to latex should be vaccinated in a latex-free environment. Most of the products currently used are synthetic (butyl, chlorobutyl, styrene-butadiene or halobutyl rubber), although a few have a type I elastomeric closure with 10% of latex.
If the symptoms reported by the child consist solely of contact allergy to latex, the patient can be vaccinated in the usual manner.10 If the previous reaction was anaphylactic, administration of the vaccine in a latex-free environment must be guaranteed.
Conflict of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: Echeverría Zudaire L, Ortigosa del Castillo L, Alonso Lebrero E, Álvarez García FJ, Cortés Álvarez N, García Sánchez N, et al. Documento de consenso sobre la actitud ante un niño con una reacción alérgica tras la vacunación o alergia a componentes vacunales. An Pediatr (Barc). 2015;83:63.